
DEPARTMENT OF THE NAVY
HEADQUARTERS UNITED STATES MARINE CORPS
3000 MARINE CORPS PENTAGON
WASHINGTON, DC 20350-3000
NAVMC 4000.2
LPC
NAVMC 4000.2
From: Commandant of the Marine Corps
To: Distribution List
Subj: MARINE CORPS CLASS VIII MANAGEMENT HANDBOOK
Ref: (a) MCO 6700.5, MEDICAL AND DENTAL (Class VIII) MATERIEL
SUPPORT OF THE MARINE OPERATIONAL FORCES
1. Purpose. The Marine Corps Class VIII Management Handbook
provides guidance to Marine Forces on the execution of MCO
6700.5, Medical and Dental (Class VIII) Materiel Support of the
Marine Operational Forces.
2. Background. The Marine Corps is responsible for fulfilling
a capability for Class VIII materiel equivalent to 60 Days of
Supply (DOS). The Class VIII Management Handbook describes the
process and procedures of the Marine Corps to manage Class VIII
materiel, to establish a surge capability equivalent to 60 DOS,
and to fulfill re-supply capability requirements equivalent to
61-180 DOS. The Handbook provides detailed guidance on actions
required to effectively execute the Class VIII management roles
and responsibilities established in MCO 6700.5.
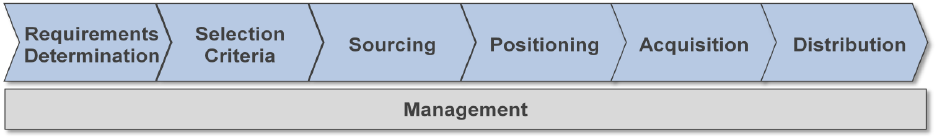
The Class Management Handbook describes the actions required to
manage the commodity through seven core functions: Management,
Requirements Determination, Selection Criteria, Sourcing,
Positioning, Acquisition, and Distribution. These functions are
consistent with the War Reserve Materiel Program, of which Class
VIII materiel is a component.
The seven core functions are also in line with the Military
Health System/Medical Logistics Enterprise processes used by
Navy Medical Logistics Command (NAVMEDLOGCOM) to determine
medical support requirements.
DISTRIBUTION STATEMENT A: Approved for public release;
distribution is unlimited.


Class VIII Management
i
NAVMC 4000.2
Marine Corps
Class VIII Management
Handbook
Class VIII Management
ii
Table of Contents
Overview......................................................iii
Management....................................................1-1
Requirements Determination ...................................2-1
Selection Criteria ...........................................3-1
Sourcing......................................................4-1
Positioning...................................................5-1
Acquisition ..................................................6-1
Distribution .................................................7-1
Marine Forces Reserve (MARFORRES).............................8-1
Systems Descriptions..........................................9-1
AMALs/ADAL Set List..........................................10-1
Acronyms & Definitions.......................................11-1
Class VIII Management
iii
Overview
The Marine Corps is responsible for fulfilling a capability for Class
VIII materiel equivalent to 60 Days of Supply (DOS). For policy level
guidance on Class VIII managements see MCO 6700.5.
This chapter describes the actions taken by the Marine Corps to manage
Class VIII materiel, to establish a surge capability equivalent to 60
DOS, and to fulfill re-supply capability requirements equivalent to
61-180 DOS. The actions will be presented in terms of how they relate
to the War Reserve Materiel (WRM) Functions, shown below.
Figure 8.1 WRM Functions
Although the WRM Functions are executed separately, they are mutually
supporting activities. Additionally, these functions are in line with
the Military Health System/Medical Logistics Enterprise processes used
by Navy Medical Logistics Command (NAVMEDLOGCOM) to determine medical
support requirements. Of note, the final Disposition phase of the
NAVMEDLOGCOM process is not represented in the WRM Functions.

Class VIII Management
1-1
NAVMC 4000.2
Class VIII Management
Management
Chapter 1
Class VIII Management
1-2
Table of Contents
A. Management ................................................1-3
1. Recurring Management Activities..........................1-3
2. Inventory Process........................................1-6
Pre-LTI Procedures.......................................1-8
Post-LTI Procedures.....................................1-18
3. Proper Care and Storage of Pharmaceuticals..............1-23
4. CBRN Materiel Management................................1-24
5. Biomedical Equipment Management.........................1-25

Class VIII Management
1-3
A. Management
The Marine Corps manages the majority of Class VIII assets at the
individual Medical Logistics Companies (MEDLOGCOs), Supply Battalion.
MEDLOGCOs are required to maintain the Authorized Medical Allowance
Lists/Authorized Dental Allowance List (AMALs/ADAL) for the respective
Marine Expeditionary Force (MEF). The authorized lists are associated
with the approved Table of Equipment (T/E) established by Deputy
Commandant Combat Development & Integration (DC CD&I) based on the
Marine Requirements Oversight Council’s approved scenarios. Although
the T/E is assigned to the MEFs, the MEDLOGCOs are the custodians for
the equipment and consumables. The MEFs can draw against available
assets held by MEDLOGCOs, as needed.
Additionally, MEFs are responsible for managing Class VIII Chemical,
Biological, Radiological, Nuclear (CBRN) materiel in collaboration
with Marine Corps Logistics Command (MCLC). MEFs maintain all Class
VIII CBRN materiel as individual line items, rather than as configured
AMALs, at the quantities required to support a force employment
package. MEFs source their Class VIII CBRN requirements through a
combination of in-stores assets or through the use of existing DLA-
managed contingency contracts. It is important to note that MEFs do
not manage CBRN decontamination kits. (See Chapter 1.4. for more
information on management of CBRN assets).
1. Recurring Management Activities
There are several additional management activities required to
maintain Class VIII materiel. The purpose and impact of these
activities are explained below.
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management
Accountability
AMALs/ADALs are organic assets to the Marine Corps and apart of the
T/E. As with other T/E items they are required to be accounted. The
Marine Corps has designated the Total Force Structure Management System
(TFSMS) as the information system to account for T/E quantities.
MEDLOGCOs are required to maintain accountability of AMALs/ADAL
inventory through Defense Medical Logistics Standard Support –
Assemblage Management Module (DMLSS-AM) and in Global Combat Support
System-Marine Corps (GCSS-MC) (or SASSY, as applicable).
MEDLOGCOs are also responsible for maintaining all records concerning
inventory adjustments and materiel dispositions for a minimum of two
years and financial records for a minimum of 6.25 years.
MEDLOGCOs will conduct a wall-to-wall inventory reconciliation of all
standardized configuration and line item AMALs/ADAL at a minimum of one
time per year. During this time, all assets will be marked as deployed
or stored in the DMLSS-AM in order to initiate the physical count of

Class VIII Management
1-4
all materiel. The current AMALs/ADAL list can be found on Medical
Logistics Online (MLO).
Reference: Visit https://ips.usmc.mil/sites/mefkb/default.aspx for
current AMALs/ADAL line list; see MCO P4400.151B for procedural
inventory and accounting guidance; and see the ―Assemblage Management
(AM) section,‖ in NAVMC 4000.3 for DMLSS assemblage management
guidance. Additionally, see DoD Financial Management Regulation Vol. 5,
Chapter 21.
Important Note: Standardly configured AMALs/ADAL will be inventoried as
a configured assemblage with supporting documentation (e.g., inventory
list, packing list, etc.)
AMALs/ADAL Configuration
AMALs/ADAL are modularly configured in standardized configurations to
minimize deviations for embarkation and transportation requirements.
In addition to the obvious benefit of optimizing cube and weight, the
AMALs/ADAL, when appropriate, are packaged to optimize employment of
health services. Based on those benefits and Global Sourcing
requirements, it is imperative that the MEDLOGCO maintain the
AMALs/ADAL in their approved configurations. Failure to configure in
the manner prescribed by Marine Corps Systems Command (MCSC) may result
in improper employment of the AMALs/ADAL to facilitate Global Sourcing
efforts.
AMALs/ADAL are issued in complete blocks only; partial blocks are not
authorized. If a requesting unit requires supplemental equipment or
consumables not provided in the standard AMALs/ADAL block, then it is
the responsibility of the requesting unit to fund and request the
additional order.
Exception: Some equipment and supplies may be maintained as line items
to facilitate stock rotation and maintenance (i.e., expiration date
management for pharmaceuticals and battery recharging for equipment).
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx; NTTP 4-
02.1, 3.3.3.4; ―Assemblage Management (AM)‖ section in NAVMC 4000.3.
Important Note: The Marine Corps standardizes across services to the
best of its ability; however, maintaining alignment with the Marine
Corps mission and mission-specific requirements receives higher
precedence than joint standardization. MCSC is responsible for service
standardization, which is conducted through the Defense Medical
Materiel Program Office (DMMPO) and Assemblage Life Cycle Management
(ALCM).
Class VIII Management
1-5
Medical Materiel Recalls
All equipment and pharmaceuticals that are under recall and/or have
problems that cannot be fixed by Bio-Medical Equipment Technicians
(BMET) will be announced via message from the US Army Medical Materiel
Agency (USAMMA) website. In special cases in which there is a risk to
life, a special Naval message and a MARADMIN will be released.
Reference: http://www.usamma.army.mil
Database Management
The Marine Corps Enterprise Software Portfolio contains Commercial Off
the Shelf (COTS), Government Off the Shelf (GOTS), and joint software
applications used within the Marine Corps Enterprise. For ease of
categorization, software applications are divided into functional
areas. The Marine Corps is considered a stakeholder in these functional
areas and is therefore responsible for maintaining a USMC software
portfolio in the Department of the Navy (DON) Application and Database
Management System (DADMS). DMLSS is included in the USMC software
portfolio and is therefore a system for which the Marine Corps is
required to maintain an Authority to Operate (ATO).
Guaranteed Returns Program
Guaranteed Returns Program is for Class VIII consumables and equipment
that are at or near their expiration date and have not had their
manufacturer seal tampered with or broken. The consumables in question
can be returned to the manufacturer for a replacement of the same
medicine or equipment.
Shelf Life Extension Program
Shelf Life Extension Program (SLEP) should be used whenever there is a
justification. All desired perishable pharmaceuticals should be
identified 210 days prior to the end of shelf life so they can be
vetted through the proper chain of command.
Reference: See Navy BUMED 6710.62A for criteria and instructions.
Class VIII Management
1-6
Figure 8.2 Class VIII Management Activities Process Descriptions
2. Inventory Process
The MEDLOGCOs are responsible for ensuring that proper equipment and
consumables are present or available to deliver the appropriate
capability of health services. Multiple management activities exist to
aid this process, including the accountability procedures, AMALs/ADAL
configuration standards, attainment reporting, capability analysis,
database management, guaranteed returns program, regular inventories,
medical materiel recalls, and Shelf Life Extension Program described
in the tables above. Maintenance and sufficient supply of equipment
and consumables are also achieved through an inventory process
facilitated by medical databases such as Defense Medical Logistics
Standard Support (DMLSS) and Medical Logistics Online (MLO), as well
as through Limited Technical Inspections (LTIs).
DMLSS provides access to the Packing List, Replenishment Process,
Assemblage Status Summary Report Criteria, and Assemblage Status
Summary Reports (see example in Chapter 1.d.), which can be used to
identify deficiencies or excess items. Additionally, upon receipt of
a line list from MCSC after Modernization Review, MEDLOGCOs use DMLSS
to generate manual DUE-INs by line item, which update as each item is
received and associate items to the appropriate blocks. Of note,
blocks that are out for exercises, operations, or deployments must be
deselected to allow for accurate updating of the system when those
blocks are returned. For additional information on how to complete
the inventory process, please see the NAVMC 4000.3 and
https://jml149.dmlss.detrick.army.mil/DMLSSU/, which offers DMLSS
training modules.
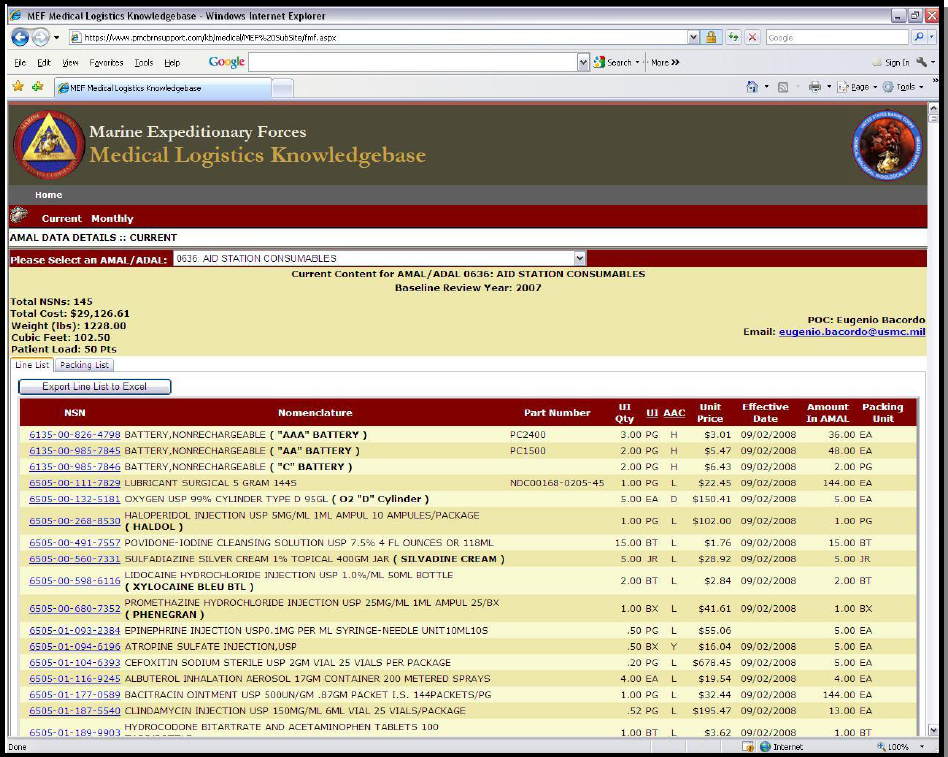
MEDLOGCOs can use MLO to view both the current list, which displays
new items or item increases from UDR updates, and the proposed list,
which shows replacements and new and increased items based on the
Modernization Review. Once items are purchased for the proposed list,
Receipts
Upon receipt of allowance materiel, custodians will check all items to
determine necessary storage requirements. Manufacturer guidance should
be followed in the storage requirements associated with medical
materiel. Environmental recommendations (i.e., temperature, humidity)
related to the storage requirements should also be followed to ensure
shelf life of the materiel (please see Chapter 1.3 for additional
information).
Reference: Regulations pertaining to security and inventory of
controlled substances, precious metals, pilferable items, and other
security type items are contained in NAVMED P117, Chapter 21, Section
2. Federal Supply Catalog information contained DoD 4100.39M, Volume 1
includes information reflecting security type items, special storage
codes, and shelf life codes to be observed.
Class VIII Management
1-7
MLO will promote from ―proposed‖ to ―current.‖ MARCORSYSCOM will then
send a spreadsheet list of items purchased to the respective MEDLOGCO
in preparation for delivery, and the MEDLOGCO will enter the purchased
item information into DMLSS as described above. MLO capabilities
allow MEDLOGCOs to view changes either in the past month or the past
six months to limit inventory workload.
For additional information regarding viewing and managing UDR and
replenishment items in DMLSS, please see the “Standard Assemblage
Update” and “Searching for Replenishment Items” sections of the NAVMC
4000.3.
a. Medical Logistics Online (MLO) AMALs/ADAL List (SAMPLE)

Class VIII Management
1-8
Additionally, Limited Technical Inspections (LTIs) ensure proper
equipment, consumable, and health service delivery. There are two
forms of LTI: Pre-LTI and Post-LTI.
Pre-LTI Procedures
Pre-LTI procedures are performed prior to any issuing of AMALs/ADAL in
order to ensure that all equipment and consumables are accounted for
and in good working order.
Participants in this phase are:
Marine Expeditionary Forces (MEFs)
Marine Forces Reserve (MARFORRES)
Marine Logistics Group (MLG)
Medical Logistics Company (MEDLOGCO)
Requesting unit
Systems in this phase are (See Chapter 9 for Systems Descriptions):
Defense Medical Logistics Standard Support—Assemblage Management
Module (DMLSS-AM)
Global Combat Support System-Marine Corps (GCSS-MC)
See the Useful Reports section in the NAVMC 4000.3, for additional
information to aid the Pre-LTI process.
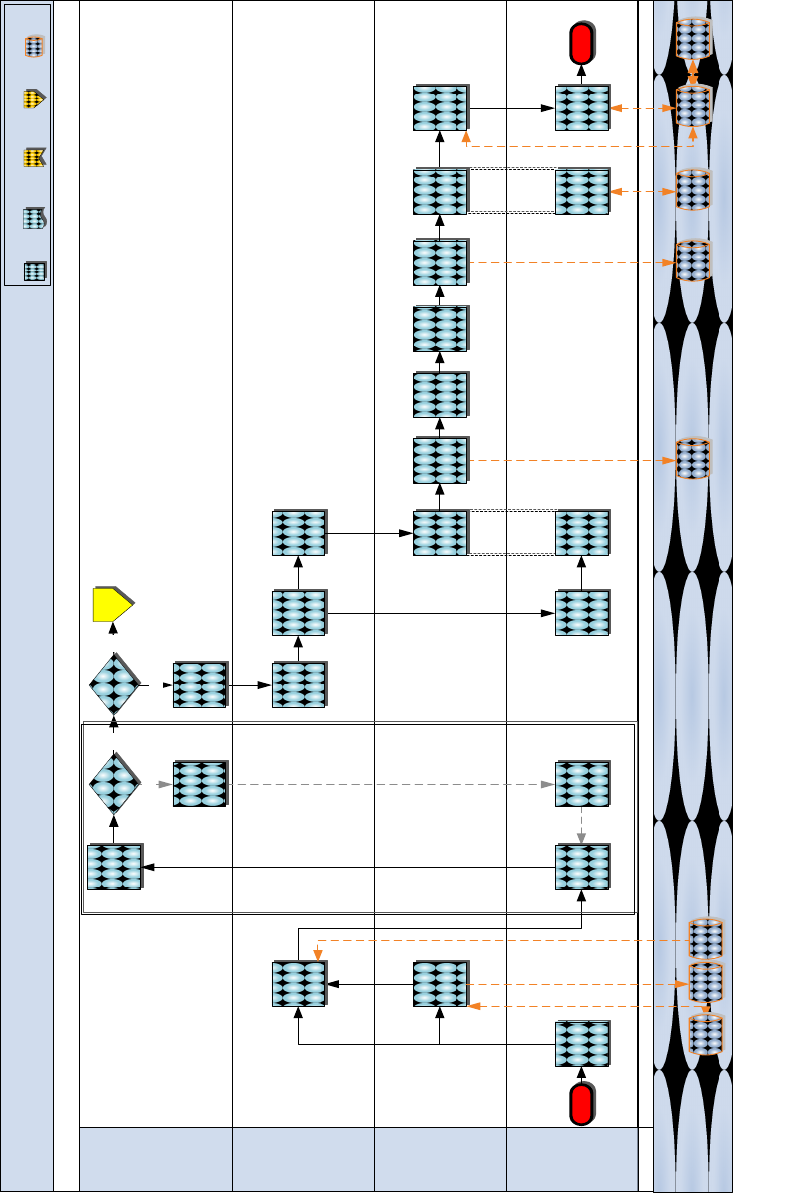
Class VIII Management
1-9
a. Process Map
Figure 8.3 Pre-LTI Process
Pre - LTI Procedures
MEDLOGCO
System
Unit
(Requesting/
On-boarding)
MSC / MLG
MEF/ MARFORRES
DMLSS-
AM
Conduct
Asset Review
Review
AMALs/ADAL
Status
Order
deficiencies
DMLSS-
AM
Coordinate to
schedule Joint
LTI
Coordinate to
schedule Joint
LTI
DMLSS-
AM
Pull AMALs/
ADAL
No
Yes
Reject
request and
send back to
Unit
Modifies
request for
resubmission
Approve?
Notify unit of
request
approval
Task
designated
office to
support
request
Task Class
VIII support
Receive task
and prepare
to provide
support
Receive
notification
SASSY
Receive
materiel
request
DMLSS-
AM
Review request
& verify the
requesting unit
rates the
materiel
Review request
& determine
AMAL/ADAL
deficiencies
Refine &
submit
materiel
requirements
request
Start
Conduct Joint
LTI )
Conduct Joint
LTI
TFSMS
This portion of the process is continuous until
request is approved
Submit
preliminary
materiel
requirement
request for
coordination
Transfer
AMALs/ADAL
to unit & drop
asset in
records
Pick-up asset
in records
GCSS-
MC
TFSMS
End
Is global
sourcing
required
No
Reference
Global
Sourcing
Process
Yes
Process
Step
Document System
Previous
Phase
Key:
Next
Phase
Class VIII Management
1-10
b. Process Step Descriptions
1. Submit preliminary materiel requirement request for coordination
Description: For pre-coordination, Requesting/Using unit submit
materiel requirements request to fulfill assets needed to support
an assigned exercise, operation, deployment, or contingency. The
request is submitted via an AMAL Request Letter. The draft AMAL
Request Letter should include AMAL quantities, supplemental
medical items, narcotics, Reporting Officer (RO), and Course of
Action (COA)*, as required. Unit coordinate with its internal
Chain of Command (Supply Battalion/CLR) to approve a draft AMAL
Request Letter and then send to the MEDLOGCO.
Important Note: Units must submit materiel requirements a minimum
of 45 days prior to the event, exercise, or deployment. The HSSE
Letter must include a Line Of Accounting (LOA).
Input: Warning Order/Letter of Instruction (LOI)
Output: Draft AMAL Request Letter
Document: See Chapter 1.c. for an example ―AMAL Request Letter‖
*COA does not apply to MARFORRES.
2. Review request & determine AMALs/ADAL deficiencies
Description: For pre-coordination, MEDLOGCO receive the draft
AMAL Request Letter from the unit and determines asset
availability/readiness by creating an assemblage roll-up report
in DMLSS. This report summarizes the readiness status of
multiple AMAL blocks and identifies the overall dollar
deficiency. MEDLOGCO incorporate the deficiency data into the
draft AMAL Request letter and send to the MSC/MLG.
Input: Draft AMAL Request Letter
Output: E-mail/phone call
3. Review request & verify the requesting unit rates the materiel
Description: For pre-coordination, MSC or MLG (HSSO), as
appropriate, receive the draft AMAL Request Letter and verifies
whether or not the unit rates the requested AMALs/ADAL blocks,
based on the Table of Equipment (T/E) established in TFSMS.
MSC/MLG incorporate the approval/ disapproval recommendation into
the draft AMAL Request Letter and send to the Requesting/Using
unit for refinement and submission.
Input: E-mail/phone call
Output: Health Service Support Element (HSSE) endorsement/draft
AMAL Request Letter

Class VIII Management
1-11
4. Refine and submit materiel requirements request
Description: Requesting/Using unit refine and submit AMAL
Request Letter to MEF/MARFORRES for approval.
Input: HSSE endorsement/draft AMAL Request Letter
Output: AMAL Request Letter (submitted by unit)
5. Receive materiel request
Description: MEF/MARFORRES receive the AMAL Request Letter and
review the input from Requesting/Using Unit. MEF/MARFORRES
determine whether or not to approve the request for the
AMALs/ADAL through a two-part approval process.
Input: AMAL Request Letter (submitted by unit)
6. Reject request and send back to Unit
Description: If MEF/MARFORRES deny the request provided in the
AMAL Request Letter, the MEF/MARFORRES send the letter back to
the unit with a justification for why the request was denied.
Input: Draft AMAL Request Letter
Output: Denied unit AMAL Request w/justification
7. Modify request for resubmission
Description: Requesting/Using unit receive the denied AMAL
Request and modify in accordance with the justification provided
by the MEF/MARFORRES and resubmit for approval.
Important Note: The resubmission of the Adjusted AMAL Request
letter will follow the same process steps as the original
submission.
Input: Denied unit AMAL Request w/justification
Output: Adjusted AMAL Request Letter
8. Task designated office to support
Description: If MEF/MARFORRES approve the request provided in
the AMAL Request Letter, the MEF/MARFORRES task the MSC/MLG to
prepare Class VIII materiel for release to the unit.
Input: Unit AMAL Request
Output: HSSE endorsement of AMAL Request + Unit AMAL Request
Letter

Class VIII Management
1-12
9. Receive task and prepare to provide support
Description: MSC or MLG (HSSO), as appropriate, receive the
approved/signed MEF endorsement and unit request and initiate
procedures required to prepare Class VIII materiel for release to
the unit.
Input: HSSE endorsement of AMAL Request + Unit AMAL Request
Letter
10. Notify unit of request approval
Description: MSC/MLG, as appropriate, notify the unit that the
AMAL Request Letter has been approved and provide instructions on
the unit’s requirements for the Pre-LTI process.
Output: Email/Phone call
11. Task designated office to support request
Description: MSC/MLG, as appropriate, task the MEDLOGCO to
prepare Class VIII materiel for release to the unit. This task
is provided in the form of a Pre-LTI Letter, which contains a
summary of the items (i.e., equipment and consumables) and is
approved for release.
Output: HSSE endorsement of AMAL Request + Unit AMAL Request
Letter
12. Receive notification
Description: Requesting/Using unit receive notification of
approval and instructions from the MSC/MLG required to initiate
the Pre-LTI process. Requesting unit coordinate internally to
designate a primary/alternate Reporting Officer to conduct the
Pre-LTI and a preferred date/time to conduct the Pre-LTI.
Important Note: The RO must be an Officer or SNCO designated from
the requesting unit.
Input: Email/Phone call
Output: Identified ROs, Proposed Pre-LTI date/time

Class VIII Management
1-13
13. Coordinate to schedule Joint LTI
Description: Requesting/Using unit and MEDLOGCO coordinate to
make final preparation for the Pre-LTI. This includes confirming
the date/time and discussing roles/responsibilities and
expectations for the Pre-LTI. Based on unit requirement, MEDLOGCO
generate Pre-LTI to include AMAL quantities, supplemental medical
items, LOA, RO, etc. Any remaining requirements prior to the
inventory are assigned accordingly.
Input: Designated RO, Proposed Pre-LTI date/time
Output: Pre-LTI Letter
14. Review AMALs/ADAL Status
Description: Prior to the Pre-LTI, MEDLOGCO run a Status Summary
Report in DMLSS-AM. This report provides the attainment
percentage and the dollar deficiency for a given block. The
report enables the MEDLOGCO to identify the appropriate block to
pull.
Input: Pre-LTI Letter/Schedule date
Output: Assemblage Status Summary Report (DMLSS-AM)
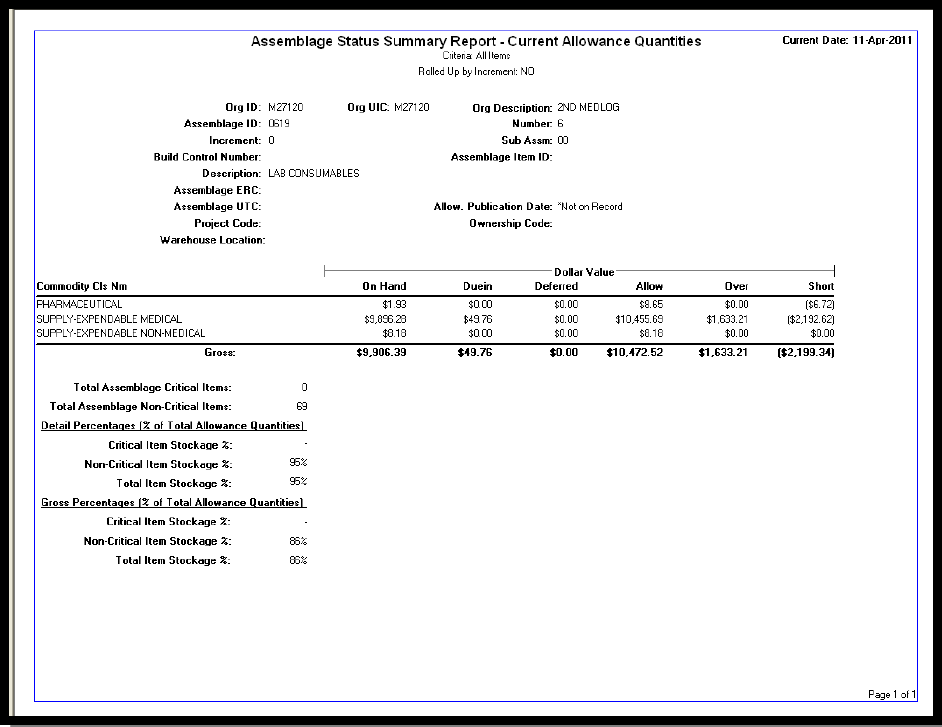
Document: See Chapter 1.d. for an example “Assemblage Status
Summary Report”
15. Pull AMALs/ADAL
Description: Based on the block identified from the Status
Summary Report, MEDLOGCO pull the block from the warehouse.
MEDLOGCO determine the deficiencies in the block requiring
replenishment.
Input: Assemblage Status Summary Report (DMLSS-AM)
Output: List of block deficiencies
Reference: See Chapter 1.d. for an example “Assemblage Status
Summary Report” and reference the ―Useful Reports‖ section of the
NAVMC 4000.3 for additional information.

Class VIII Management
1-14
16. Conduct Asset Review
Description: MEDLOGCO conduct an asset review to determine in-
house (on-hand) replenishment from secondary location or excess
stocks in the warehouse that can be used to increase the block’s
readiness percentage. MEDLOGCO pull shelf-life & other items to
build AMALs/ADAL. Then, MEDLOGCO adjust the deficiencies to
reflect items required for external replenishment.
Input: Block deficiencies list (DMLSS-AM, ―Replenishment
Report‖)
Output: Adjusted block deficiencies list
Reference: ―Searching for Replenishment Items‖, ―Useful
Reports‖, and ―Asset Review‖ sections in the NAVMC 4000.3.
17. Order deficiencies
Description: MEDLOGCO utilize DMLSS to order remaining
deficiencies not available in the warehouse. DMLSS sources
deficiencies from either the Prime Vendor Medical Surge
(PVM)/Prime Vendor Pharmacy (PVP) or through DLA’s Contingency
Contracts. (See Chapter 4.2. Sourcing Timeline for amplifying
information on PVM/PVP and DLA contracts). MEDLOGCO generate a
―Replenishment Report,‖ a list of items to be procured to
increase readiness, and a DUE-INs Report, a list of what is
coming in based on orders, via DMLSS.
Important Note: Orders pending in DMLSS are reflected as DUE-INs.
Once the orders are processed, the percentage readiness is
automatically adjusted in the AMALs/ADAL block. Therefore, it is
essential that MEDLOGCO accounts for planned verse actual
adjustments to block inventory to ensure they are not conducting
unnecessary inventories or reporting false attainment. An
example checklist is provided in Chapter 1.d. to facilitate
tracking of external replenishment.
Due to delivery times associated with PVM/PVP, it may take as few
as 48 hrs and up to 14 days to get the requested assets required
to bring the block up to 100%.
Input: Adjusted block deficiencies list
Output: Replenishment Report & DUE-INs Report
Reference: ―Useful Reports‖ section in NAVMC 4000.3.
Class VIII Management
1-15
Figure 8.4 Pre-LTI Process Descriptions
18. Conduct Joint LTI
Description: Once all assets are acquired to bring the block up
to the desired level of readiness, the MEDLOGCO and designated
unit RO conduct a Joint LTI to include a QA/QC of line items,
item quantity, SL3 equipment, and shelf-life assets and ensure
that Biomedical has conducted operational checks on designated
equipment. A Controlled Substance Officer is present during this
process to sign for all narcotics. Upon completion of a
satisfactory inspection, RO sign for the AMALs/ADAL and
acknowledge the percentage of readiness and standardized
configuration or most current packing list from MCSC.
Important Notes: The RO must be an Officer or SNCO designated
from the requesting unit. Additionally, narcotics/controlled
substances must be signed for by an Officer in accordance with
MANMED P-117 and OPNAVINST 3120.32C.
The Joint LTI does not apply in cases of global sourcing or for
the release of MARFORRES assets, in which case only an LTI is
performed. Per MARFORRES Force Order 6000, MARFORRES will
conduct a JLTI if the requesting unit funds MARFORRES travel.
Input: Confirmed Pre-LTI date/time, MCSC Packing List
Output: Signed AMALs/ADAL Block; Pre-LTI Packing List
19. Transfer AMALs/ADAL to unit & drop asset in records
Description: Once the RO has signed for the AMALs/ADAL, MEDLOGCO
coordinate with unit to transport asset. Upon completion,
MEDLOGCO remove the asset from the MEDLOGCO property records in
GCSS-MC (or SASSY, as applicable). Additionally, MEDLOGCO update
DMLSS-AM of all DUE-INs receipts and freeze AMALs/ADAL block at
the level at which it was transferred.
Reference: http://www.marcorsyscom.usmc.mil/sites/gcss-
mc/index.aspx; ―Transferring an Assemblage Out of Your
Organization (Loss)‖ section in NAVMC 4000.3.
Input: Signed AMALs/ADAL Block
Output: AMALs/ADAL GCSS-MC record
20. Pick-up asset in records
Description: Upon receipt of the AMALs/ADAL, Requesting/Using
unit add the asset in the unit’s property records in GCSS-MC (or
SASSY, as applicable).
Reference: http://www.marcorsyscom.usmc.mil/sites/gcss-
mc/index.aspx; ―Transferring an Assemblage into Your Organization
(Gain)‖ section in NAVMC 4000.3.
Input: AMALs/ADAL GCSS-MC record

Class VIII Management
1-16
c. Example: AMAL Request Letter (SAMPLE)
From: Commanding Officer, [Requesting Unit]
To: Commanding Officer, Medical Logistics Company, [1
st
, 2
nd
, or 3
rd
, Delete
for MFR] Supply Battalion, [1
st
, 2
nd
, 3
rd
, 4
th
] Marine Logistics Group
[Location]
Via: Commanding General, [1
st
, 2
nd
, 3
rd
] Marine Expeditionary Force
(MEF)/Marine Forces Reserve (MFR)
Commanding General, [1
st
, 2
nd
, 3
rd
, 4
th
] Marine Logistics Group or Major
Subordinate Command, G-3 (HSSE)
Subj: REQUEST FOR AMALs/ADAL IN SUPPORT OF FIELD OPERATIONS.
1. It is requested that the following AMALs/ADAL, pharmaceutical, and
consumable list be provided in support of Field Operations.
NOMENCLATURE QTY
[Block, Designate AMAL/ADAL] [xxx]
EXAMPLE: 699 AMAL BLOCK 01
2. The following additional information is provided
a. Responsible Officer (RO): Rank, Last, First, Middle Initial. xxx-xx-
last four of SSN] EXAMPLE: LT John , Doe xxx-xx- [
b. Date of inventory/ LTI: [dd_MMM-yyyy] EXAMPLE: 29 APR 2010
c. Date of pick up: [dd_MMM-yyyy] EXAMPLE: 29 APR 2010
d. Estimated date of return: [dd_MMM-yyyy] EXAMPLE: 15 MAY 2010
e. RA Job Order Number (JON): [JON associated with line of accounting to
fund request]
f. Reporting Unit (RUC): [xxxxx] EXAMPLE: 11330
3. Point of contact for this request is, [Rank, Last, First, Middle
Initial., Phone, Email] EXAMPLE: HM3 Vollstedt, Ross @(760) 830 5552
4. An advance copy of this request was sent to CO, Medical Logistics
Company, 1
st
Supply Battalion, 1
st
Marine Logistics Group on 13 APR 2010.
J. A. SMITH
Class VIII Management
1-17
d. Assemblage Status Summary Report (SAMPLE)

Class VIII Management
1-18
Post-LTI Procedures
Post-LTI procedures are performed after the return of the using unit
in order to ensure proper configuration (according to MCSC standards),
validate that all equipment is accounted for and in good working
order, and determine preliminary replenishment requirements.
It is important that all AMALs/ADAL are returned in the same
configuration in which the equipment was issued (i.e., cleaned and in
the standard configuration as Pre-LTI).
Participants in this phase are:
Marine Logistics Group (MLG)
Medical Logistics Company (MEDLOGCO)
Supported unit
Systems in this phase are (See Chapter 9 for Systems Descriptions):
Defense Medical Logistics Standard Support—Assemblage Management
Module (DMLSS-AM)
Global Combat Support System-Marine Corps (GCSS-MC)
Standard Accounting, Budgeting, and Reporting System (SABRS)
Class VIII Management
1-19
a. Process Map
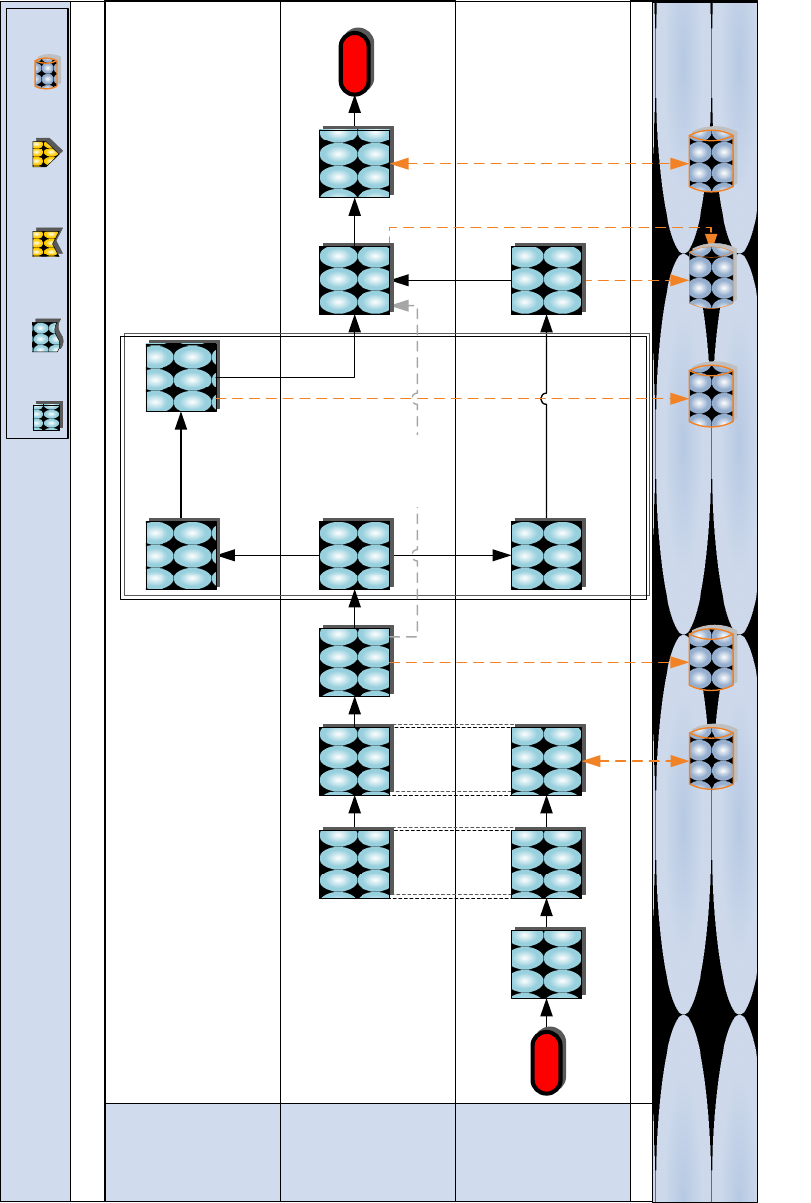
Figure 8.5 Post-LTI Process
Post-LTI Procedures
Unit
(Supported)
MEDLOGCO
MLG
(Comptroller)
Review cost
and prepare
to allocate
funds*
System
Order items to
replenish
stocks
End
* These steps do not apply
to MARFORRES
DMLSS-
AM
Create and
submit cost
for consumed/
broken items*
Conduct Joint
LTI
Review
AMALs/ADAL
Status
Coordinate to
schedule Joint
LTI
Conduct Joint
LTI
Charge unit’s
line of
accounting*
Return from
exercise,
operation,
deployment, or
contingency
Start
SABRS
Coordinate to
schedule Joint
LTI
DMLSS-
AM
Review cost
for assets
used/ items
needing to be
reconstituted*
MARFORRES
DRAFT
DMLSS-
AM
GCSS-
MC
Drop asset in
records
Pick-up asset
in records
System
Key:
Process
Step
Document
Previous
Phase
Next
Phase
Class VIII Management
1-20
b. Process Step Descriptions
1. Return from exercise, operation, deployment, or contingency
Description: Supported unit return from exercise, operation,
deployment, or contingency and report to MEDLOGCO no more than
one week following return to initiate coordination for a Post-
LTI. Supported unit designate a primary/alternate Reporting
Officer to conduct the Post-LTI and a preferred date/time to
conduct the Post-LTI. If AMALs are not returned in a timely
fashion, units may be charged for the total cost of the AMAL.
Important Note: The RO must be an Officer or SNCO designated from
the requesting unit. The RO must be the same for both Pre- and
Post-LTI.
Output: Designated ROs, Proposed Post-LTI date/time
2. Coordinate to schedule Joint LTI
Description: Unit and MEDLOGCO coordinate to make final
preparation or the Post-LTI. This includes confirming the
date/time and discussing roles/responsibilities and expectations
for the Joint LTI.
Input: Proposed Post-LTI date/time
Output: Confirmed Post-LTI date/time
3. Conduct Joint LTI
Description: MEDLOGCO and designated unit RO conduct a Joint LTI
to include; a QA/QC of line items, item quantity, SL3 equipment,
shelf-life assets, and ensure that Biomedical has conducted
operational checks on designated equipment. A Controlled
Substance Officer is present during this process to sign for all
narcotics. Upon completion of a satisfactory inspection,
MEDLOGCO sign for the AMALs/ADAL and acknowledge the percentage
of readiness.
Important Notes: The RO must be an Officer or SNCO designated
from the requesting unit. Additionally, narcotics/controlled
substances must be signed for by an Officer in accordance with
MANMED P-117 and OPNAVINST 3120.32C.
The Joint LTI does not apply in cases of Global Sourcing or for
the release of MARFORRES assets, in which case only an LTI is
performed. Per MARFORRES Force Order 6000, MARFORRES will
conduct a JLTI if the requesting unit funds MARFORRES travel.
Input: Confirmed Post-LTI date/time, Pre-LTI Packing List

Class VIII Management
1-21
4. Review AMALs/ADAL Status
Description: MEDLOGCO determine items needing replenishment
(broken/consumed) and update in DMLSS. Then MEDLOGCO run a
Status Summary Report in DMLSS and obtain the attainment
percentage and the dollar deficiency for the returned block. The
report enables the MEDLOGCO to identify the line items and
equipment to order to bring the block up to desired readiness.
Output: Status Summary Report + Supplemental SL3 or Components
Report
Reference: ―Useful Reports‖ section in NAVMC 4000.3.
5. Create and submit cost for consumed/broken items*
Description: MEDLOGCO create a Reconciliation Letter, which
includes validation of the Pre-LTI Packing List, a summary of the
items the unit signed for, and the items that were returned.
MEDLOGCO (HSSO) send the Pre/Post-LTI Letter to the Supported
Unit and the MSC/MLG Comptroller.
Important Note: This does not apply to MARFORRES.
Input: Status Summary Report + Supplemental SL3 or Components
Report
Output: Reconciliation Letter
6. Review cost for assets used/ items needing to be reconstituted*
Description: MSC/MLG (Comptroller) receive the Reconciliation
Letter and review the cost associated with consumed/broken
assets.
Important Note: This does not apply to MARFORRES.
Input: Reconciliation Letter
Output: Replenishment Cost
7. Charge unit’s line of accounting*
Description: MSC/MLG (Comptroller) charge the Line of Accounting
(LOA) provided by the requesting unit in the AMAL Request Letter
submitted during the Pre-LTI process in accordance with local
policy (see Chapter 1).
Important Note: This does not apply to MARFORRES.
If a unit’s deployment spans over two Fiscal Years (FYs) then the
requiring unit is required to provide an updated Line of
Accounting (LOA) to the MEDLOGCO.
Input: Replenishment Cost
Output: LOA Charge
Class VIII Management
1-22
8. Review cost and prepare to allocate funds*
Description: Supported unit receive the Reconciliation Letter,
review the cost associated with consumed/broken assets, and
prepare for funds to be allocated.
Important Note: This does not apply to MARFORRES.
Input: Status Summary Report
Output: Reconciliation Letter
9. Drop asset in records
Description: Upon review of the charges, supported unit remove
the asset from its property records by TAMCN in GCSS-MC.
Reference: http://www.marcorsyscom.usmc.mil/sites/gcss-
mc/index.aspx; ―Transferring an Assemblage Out of Your
Organization (Loss)‖, NAVMC 4000.3.
Input: Reconciliation Letter
Output: AMALs/ADAL GCSS-MC record
10. Pick-up asset in records
Description: MEDLOGCO add the asset in the unit’s property
records by TAMCN in GCSS-MC.
Reference: http://www.marcorsyscom.usmc.mil/sites/gcss-
mc/index.aspx; ―Transferring an Assemblage into Your Organization
(Gain)‖, NAVMC 4000.3.
Input: AMALs/ADAL GCSS-MC record
Class VIII Management
1-23
Figure 8.6 Post-LTI Process Descriptions
c. Exceptions
There are instances when a unit returning from an operation will leave
the medical materiel, which is signed for, in-theater for other units
to fall-in on. In this case, no Post-LTI will be performed by the
departing unit, but it is recommended that the fall-in unit conduct a
Post-/Relief LTI in order to account for all equipment and materiel.
The fall-in unit will follow the Pre-LTI procedures for routing an
AMAL Request Letter up the Chain of Command. The AMAL Request Letter
will be used to report the materiel as a combat loss.
Once the MEFs sign the AMAL Request Letter and provide it to the
MSC/MLG and the MEDLOGCO, the MEDLOGCO will drop the AMALs/ADAL in
DMLSS and update the on-hand quantities in GCSS-MC. Then the MEDLOGCO
will create a Combat Loss Letter summarizing the assets for which the
unit signed. A Responsible Officer (RO), an Officer, or SNCO
designated by the unit will report to the MEDLOGCO to sign the Combat
Loss Letter. The signed letter will be provided to the comptroller at
the MLG to receive funding to replenish the stocks left in-theater.
The MEDLOGCO is responsible for replenishing the stocks after receipt
of funding.
3. Proper Care and Storage of Pharmaceuticals
All pharmaceuticals should be stored in a temperature-controlled
environment, in accordance with manufacturer guidance. Failure to
store pharmaceuticals at appropriate temperatures in combination with
regular monitoring temperature and humidity (T/H) monitoring may
result in advanced deterioration or reduced pharmaceutical
11. Order items to replenish stocks
Description: MEDLOGCO utilize DMLSS to order replenishment
stocks to bring the asset to the required level of readiness.
DMLSS sources deficiencies from either the PVM/PVP or DLA’s
Contingency Contracts. (See Chapter 4, Sourcing Timeline for
amplifying information on PVM/PVP and DLA contracts). MEDLOGCO
generate a Replenishment Report, a list of items to be procured
to increase readiness, and a DUE-INs Report, a list of what is
coming in based off order, via DMLSS.
Important Note: Orders pending in DMLSS are reflected as DUE-INs.
Once the orders are processed, the percentage readiness is
automatically adjusted in the AMALs/ADAL block. Therefore, it is
essential that MEDLOGCO accounts for planned verse actual
adjustments to block inventory to ensure they are not conducting
unnecessary inventories or reporting false attainment. Due to
delivery times associated with PVM/PVP, it may take as few as 48
hrs and up to 14 days to get the requested assets.
Output: Replenishment Report & DUE-INs Report
Reference: ―Search for Replenishment Items‖, NAVMC 4000.3.
Class VIII Management
1-24
effectiveness. Proper storage in a temperature-controlled storage
facility will ensure the shelf-life of all medical and dental
pharmaceuticals, as well as limit loss of inventory and adverse
clinical effects caused by improper storage. In order to ensure that
proper storage standards are met, all USMC units storing
pharmaceuticals will provide the following:
a. A temperature and humidity (T/H) controlled monitoring program to
ensure that all stored pharmaceuticals meet the manufacturer
requirements.
b. Continuous or periodical monitoring of stored equipment and
consumables. If monitored periodically, consumables/equipment
will be recorded twice within a 24-hour period, and all readings
will be posted and available for inspections. Continuous
monitoring will be conducted in accordance with an approved USMC
monitoring system. Use of a commercial alarm to indicate a
single event and/or record detailed time and temperature history
is recommended.
Any pharmaceuticals that have been exposed in an uncontrolled
environment for 72 hours will be considered unserviceable. The lot
number will be recorded and pulled from the shelves, and if the
pharmaceuticals have a total value in excess of $1000, they may be
submitted to the FDA for acceptance into the Shelf Life Extension
Program in accordance with BUMED 6710.62A. Pharmaceuticals that do
not meet the standards for entry into the Shelf Life Extension Program
will be prepared for disposal.
4. CBRN Materiel Management
CBRN skin decontamination kits are Class VIII assets are centrally
managed with all Marine Corps non-medical CBRN equipment. The WRM
requirements for CBRN skin decontamination kits are calculated and
managed using the same procedures as Class II materiel and are
procured by MCSC through the Warfighting PEB.
In addition to the skin decontamination kits, MEFs maintain all other
Class VIII CBRN materiel as individual line items, rather than as
configured AMALs, at the quantities required to support a force
employment package. MEFs shall determine an appropriate personnel
requirement in accordance with the minimum number of on-hand CBRN
equipment they maintain. This will be the quantiiy held on-hand at the
MEDLOGCO vice the total AAO. This number is updated on an annual
basis in order to reflect changes in MEF-held CBRN equipment levels.
MEFs source their Class VIII CBRN requirements through a combination
of in-stores assets (on-hand) or through the use of existing DLA-
managed contingency contracts (remaining AAO). MEDLOGs are required to
utilize DLA contracts for tiered resupply of remaining materiel
requirements. Additionally, MEFs are required to develop and implement
an incremental procurement and replenishment program in order to
ensure shelf life dating for MEF-held CBRN materiel is spread across

Class VIII Management
1-25
multiple manufacturer production lots. MEDLOGCOs shall follow
BUMEDISNT 6710.62 and DODI 6430.02 on DoD/Food and Drug Administration
(FDA) Shelf Life Extension Program (SLEP) materiel and shall enter all
qualified NSN in the SLEP database located at:
https://slep.dmsbfda.army.mil/portal/page/portal/SLEP_PAGE_GRP/SLEP_HO
ME_NEW.
Marine Corps Logistics Command (MCLC) is responsible for managing
Class VIII CBRN requirements to support follow-on forces and
MARFORRES, as well as managing all USMC Class VIII (SLEP) materiel.
CBRN materiel requirements managed by MCLC are sourced through a
combination of contingency contracts, DoD/FDA SLEP materiel, or in-
stores inventory. Any MEF-held Class VIII CBRN materiel that expires
and meets the criteria for testing in the DoD/FDA SLEP should be
transferred to MCLC and held as SLEP materiel.
5. Biomedical Equipment Management
Biomedical Equipment Technicians (BMETs) are taught the basic theory
and concepts of different classes of medical equipment at the DoD
Biomedical Equipment Technician (BMET) School and are capable of
performing level II maintenance on the classes of equipment held
within the Marine Corps inventory. Since some equipment is a product
of new technology, BMETs should apply their training to new equipment
with use of service and operator manuals.
Training in medical equipment technology that is not provided by the
DoD BMET School will be funded by MCSC. BMETs, in turn, will be
required to train other BMETs in the MEDLOG on new equipment in order
to sustain technical knowledge gained from initial training.
Additional training requested after the initial training class will be
funded by the requesting command.
The frequency of scheduled medical equipment maintenance should comply
with the manufacturer’s requirements or frequency established by
NAVMEDLOGCOM. The Biomedical Engineering Division (BIOMED) is
required to use the DMLSS Device Code and Life Expectancy Table for
assigning risk levels and maintenance frequency.
For additional information, please see the NAVMEDLOGCOM Biomedical
Equipment Division Support website at
https://gov_only.nmlc.med.navy.mil/.

Class VIII Management
2-1
NAVMC 4000.2
Class VIII Management
Requirements Determination
Chapter 2
Class VIII Management
2-3
A. Requirements Determination
Requirements Determination, in conjunction with the Selection Criteria
function described in the previous chapter, parallels the Plan Health
Service Support phase of the NAVMEDLOGCOM process for determining
medical support requirements.
The Marine Corps has two distinct phases for determining Class VIII
materiel requirements. The first phase is to establish initial issue
(15 DOS) requirements as a part of the unit T/E listed in TFSMS. The
AMALs/ADAL types and quantities in the T/E comprise the unit’s basic
allowance, which the MEDLOGCO will hold and maintain. These
AMALs/ADAL also undergo routine reviews to ensure the type and
quantity of materiel is in accordance with changing mission objectives
or force structure.
1. Requirements Determination — Initial Issue
Participants in this phase are:
Deputy Commandant Combat Development & Integration (DC CD&I)
Combat Development Directorate (CDD)/Logistics Integration
Division (LID)
Deputy Commandant Combat Development & Integration (DC CD&I)
Total Force Structure Division (TFSD)
Marine Corps Systems Command (MCSC)
Marine Forces (MARFOR)
Marine Expeditionary Forces (MEFs)
Marine Forces Reserve (MARFORRES)
Using unit
Additional participants that contribute to this phase include:
Marine Corps Warfighting Laboratory (MCWL)
Training & Education Command (TECOM)
MCWL coordinates with DC CD&I to support pre-phase efforts in
informing DC CD&I on any projects researching developmental
equipment/assets that may be used to fill a gap in requirements.
Additionally, MCWL supports post-phase efforts in conducting testing
and evaluation on requirements to document justification for
prioritization of requirements.
TECOM coordinates with DC CD&I to support the post-phase effort in
identifying the training requirements associated with permanent
programs.
Systems in this phase are (See Chapter 9 for Systems Descriptions):
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management
Class VIII Management
2-4
Total Force Structure Management System (TFSMS)
Global Combat Support System-Marine Corps (GCSS-MC)
Program Budgeting Documentation Database (PBDD)
a. Process Map
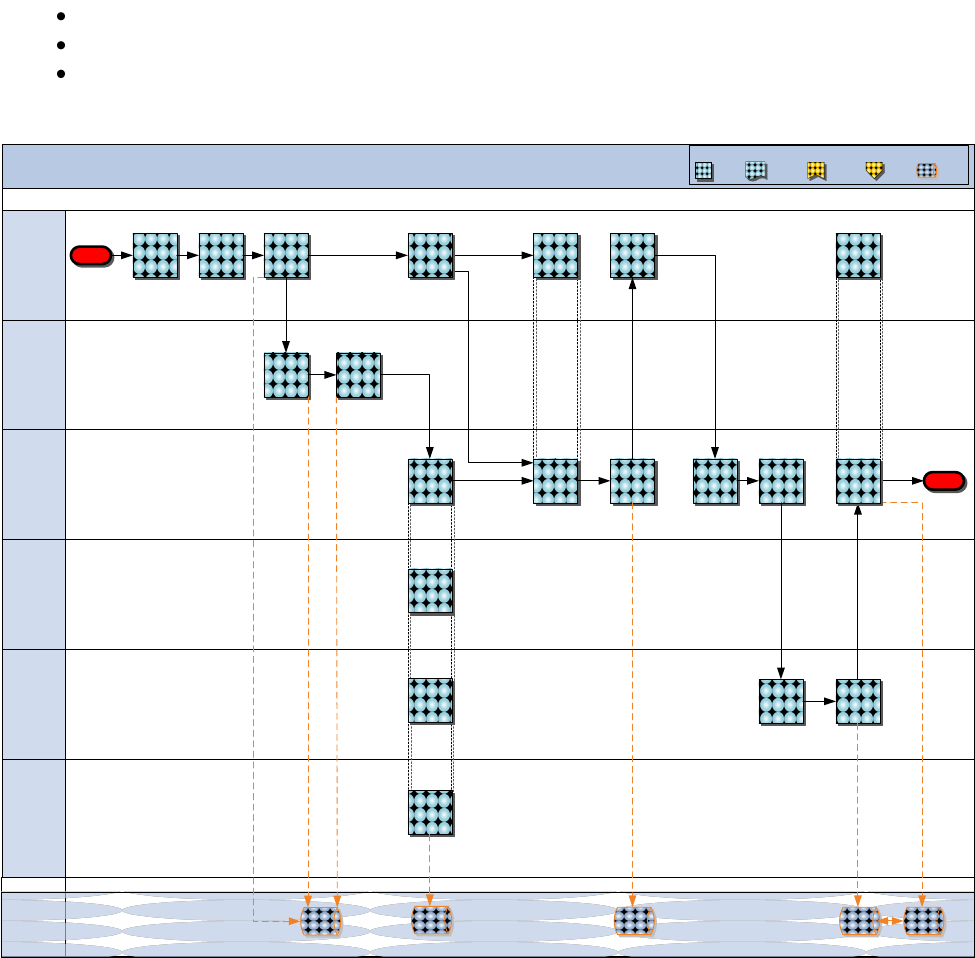
Figure 8.10 Requirements Determination—AAO Initial Issue Process
Requirements Determination – Initial Issue (AAO)
MCSC
DC CD&I
(TFSD)
Unit
MEF/
MARFORRES
(MLG)
MARFOR
DC CD&I
(CDD/LID)
System
Start
Staff
requirement
internally for
CDD approval
Inputs
requirements
into the
system of
record
Coordinate for
AAO
programming/
budgeting
Coordinate for
AAO
programming/
budgeting
View
modifications
to the AAO
View
modifications
to the AAO
TFSMS
Create &
submit
TOECR
Review &
approve
TOECR
View
modifications
to the AAO
View
modifications
to the AAO
Submit
initiative for
Class VIII
funding
Advocate for
Class VIII
funding
Receive
funding &
procure Initial
Issue assets
Field Class
VIII Initial
Issue
Receive Initial
Issue
Acknowledge
receipt of
Initial Issue
PBDD
GCSS-
MC
TFSMS
DRAFT
Determine
requirement
for initial issue
(AAO T/E)
Send
approved
SON/UUNS
End
TFSMS
View initial
Issue has
been received
Validate initial
Issue has
been received
Previous
Phase
Next
Phase
Process
Step
Document
Key:
System
Class VIII Management
2-5
b. Process Step Descriptions
1. Determine requirement for initial issue (AAO T/E)
Description: DC CD&I (CDD/LID) determine a new requirement for
initial issue (AAO T/E, 15 DOS). Based on this requirement, LID
create a Statement of Need (SON) or an Urgent Universal Needs
Statement (UUNS) to substantiate the requirement. The SON/UUNS
contain the asset’s concept of employment, characteristics, and
Approved Acquisition Objective (AAO) broken down by MEFs,
MARFORRES, Maritime Prepositioning Ships (MPS), MARSOC, Support
Establishment, and MARFORs.
Important Note: The pharmacy is not included in the initial issue
(AAO T/E, 15 DOS) process. It is the responsibility of the
MEDLOGCOs to procure through the Prime Vendor Pharmacy (PVP) or
DLA Contingency Contracts.
Input: JCIDS/EFDS Program of Records
Output: SON/UUNS
2. Staff requirement internally for CDD approval
Description: DC CD&I (CDD/LID) staff the SON/UUNS internally
through CDD to get approval for the modification to the (AAO
T/E). Approval is granted via a CDD Letter of Approval.
Input: SON/UUNS
Output: CDD Letter of Approval
3. Create and submit TOECR
Description: DC CD&I (CDD/LID) create and submit a Table of
Organization and Equipment Change Request (TOECR) to the Total
Force Structure Management Structure (TFSMS). After inputting
the TOECR into TFSMS, CDD/LID should submit the TOECR to DC CD&I
(TFSD) for review and approval.
Input: CDD Letter of Approval
Output: TOECR
4. Review and approve TOECR
Description: DC CD&I (CDD/LID) coordinate with DC CD&I (TFSD) to
determine the impacts on the Marine Corps Approved Acquisition
Objective (AAO). Upon successful completion of analysis, CDD
endorse and LID forward to TFSD for action.
Input: TOECR
Output: CDD Endorsement/Approved TOECR

Class VIII Management
2-6
5. Input requirements into the system of record
Description: DC CD&I (TFSD) input new initial issue (15 DOS)
requirements (T/E), in accordance with the approved TOECR, into
the system of record, TFSMS. Each new requirement is associated
with a Unit Identification Code (UIC).
Input: Approved TOECR
Input: Adjust T/E
6. View modifications to the AAO
Description: MCSC, MARFOR, MEF/MARFORRES (MLG), and the supported
unit view adjustments to the T/E associated with their UIC in
TFSMS.
Input: Adjust T/E
7. Send approved SON/UUNS
Description: DC CD&I (CDD/LID) send the approved SON/UUNS to
MCSC to provide MCSC with a confirmation that the AAO has been
loaded and enable MCSC to initiate procurement/budgeting.
Output: SON/UUNS
8. Coordinate for AAO programming/budgeting
Description: DC CD&I (CDD/LID) and MCSC (PM-CSE) collaborate to
generate a justification and support documentation to establish a
Class VIII initial issue (15 DOS) initiative for
programming/budgeting.
Input: SON/UUNS
Output: Initial Issue Funding Line
9. Submit initiative for Class VIII funding
Description: MCSC (PM-CSE) submit a request for Class VIII
initial issue (15 DOS) funding in the PBDD. The initiative
articulates the unfunded requirements, identifies the pre-
existing budget-line/funding profile which supports the
requirements, and justifies the need for the initial issue (15
DOS) type and quantity.
Input: Initial Issue Funding Line
Output: PBDD Initial Issue Entry

Class VIII Management
2-7
10. Advocate for Class VIII funding
Description: DC CD&I (CDD/LID) serve as the Class VIII program
advocate and articulate the value/benefit of new program
resources as outlined in the Initial Issue Initiative, in order
to help ensure that Class VIII Initial Issue (15 DOS)
requirements receive funding in the POM Process (See NAVMC
4000.1, Chapter 8: Acquisition for more information on the POM
Process). Class VIII requirements are briefed to the Warfighting
Program Evaluation Board (WIPEB).
Input: PBDD Initial Issue Entry
Output: WIPEB Submission
11. Receive funding and procure initial issue assets
Description: At the completion of the POM cycle, MCSC (PM-CSE)
receive Class VIII funding and procure initial issue (15 DOS)
assets.
Input: Class VIII funding
Output: Initial Issue
12. Field Class VIII initial issue
Description: Once MCSC (PM-CSE) receive the Initial Issue (15
DOS) assets from the procurement source, MCSC (PM-CSE) field the
assets to the designated MEF/MARFORRES (MLG).
Output: Initial Issue
13. Receive initial issue
Description: MEF/MARFORRES (MLG) receive initial issue (15 DOS)
from MCSC (PM-CSE) and incorporate into the Class VIII inventory.
Input: Initial issue
14. Acknowledge receipt of initial issue
Description: MEF/MARFORRES (MLG) add assets to Class VIII
inventory by updating the associated records in GCSS-MC (or
SASSY, as applicable). Upon completion, compare GCSS-MC records
to TFSMS and officially acknowledge receipt of initial issue (15
DOS) in TFSMS by updating the on-hand quantity for each
respective Unit Identification Code (UIC).
Output: Updated GCSS-MC record, Updated TFSMS On-hand quantity
Reference: http://www.marcorsyscom.usmc.mil/sites/gcss-
mc/index.aspx
15. Validate initial issue has been received

Class VIII Management
2-8
Figure 8.11 Requirements Determination—AAO Initial Issue Activities
Process Descriptions
It is important to note that modifications may be made to the basic
allowance by the unit to gain an additional Warfighting capability
deemed critical to operating forces for combat or contingency
operations. After they receive MEF Commander approval, units may
recommend substitutions or additions to their AMALs/ADAL T/E through
the Urgent Needs Process (UNP). Details regarding this process can be
referenced in MCO 3900.17, the Marine Corps Urgent Needs Process (UNP)
and the Urgent Universal Needs Statement (UUNS) or at
https://www.mccdc.usmc.mil.
2. Requirements Determination — Surge
There are two components that go into the development of surge
requirements for Class VIII materiel: Casualty Estimations (CASEST)
and Patient Streams.
The CASEST is a service requirement to determine the number of
casualties that are estimated in accordance with a given OPLAN. This
is an internal Marine Corps process comprised of collaboration between
PP&O (PLN) and I&L (LPC). As depicted in the Surge Requirement
process map (Section A.2.a.), LPC will request PLN’s support to
determine CASEST. In this process, PLN will review the numbered
OPLANs and derive an estimated number of casualties based on the
environment, duration, and intensity pertaining to the OPLAN. PLN and
LPC will establish CASEST based on two combat phases, three levels of
intensity, and other casualties.
Two phases of combat:
Assault (1st 30 days of combat)
Sustainment (Every subsequent 30 day period)
Three intensities of combat:
Low Intensity Conflict (LIC): Political-military confrontation
between contending states or groups below conventional war and
above the routine, peaceful competition among states. It
frequently involves protracted struggles of competing principles
and ideologies. LIC ranges from subversion to the use of means
employing political, economic, informational, and military
instruments to include irregular warfare scenarios. LICs are
Description: DC CD&I (CDD/LID) validate that initial issue (15
DOS) has been received and input into TFSMS by ensuring that on-
hand quantities for each respective UIC are appropriated updated.
Input: Updated TFSMS On-hand quantity
Output: Validated TFSMS On-hand quantity
Reference: https//:tfsms.mccdc.usmc.mil

Class VIII Management
2-9
often localized, generally in the third-world, but contained
regional and global security implications.
Medium Intensity Conflict (MIC): A Medium Intensity Conflict is
characterized by the protracted employment of regular armed
forces in combat as a major manifestation of power by the threat
and responding nations, and the designation of military
objectives to achieve political and economic goals. It may
include some or all of the techniques and characteristics of low
intensity conflict.
High Intensity Conflict (HIC): The relatively unconstrained use
of power by one or more nations to gain or protect territory and
interests that directly affects the survival of the nation. The
form of conflict is characterized by extreme levels of violence.
The employment of the full range of military force sustained by
the preponderance of other national resources to achieve military
and political victory is the primary use of nuclear weapons and
may include some or all of the characteristics of LIC and MIC.
LPC will provide these estimates to the Naval Health Research Center
(NHRC) for the development of patient streams. NHRC will divide the
casualty estimations into one of five sub-categories:
Wound in Action (WIA): A casualty category applicable to a
hostile casualty, other than the victim of a terrorist activity,
who has incurred an injury due to an external agent or cause. The
term encompasses all kinds of wounds and other injuries incurred
in action, whether there is a piercing of the body, as in a
penetration or perforated wound, or none, as in the contused
wound. These include fractures, burns, blast concussions, all
effects of biological and chemical warfare agents, and the
effects of an exposure to ionizing radiation or any other
destructive weapon or agent. The hostile casualty’s status may be
categorized as ―very seriously ill or injured,‖ ―seriously ill or
injured,‖―incapacitating illness or injury,‖ or ―not seriously
injured.‖ Also called WIA. See also casualty category.
Disease Non-Battle Injury (DNBI): A person who is not a battle
casualty but who is lost to the organization by reason of disease
or injury, including persons dying of disease or injury, by
reason of being missing where the absence does not appear to be
voluntary, or due to enemy action or being interned. Also called
DNBI casualty.
Non-Battle Injury (NBI): A person who becomes a casualty due to
circumstances not directly attributable to hostile action or
terrorist activity. Also called NBI.
Died of Wounds Received in Action (DWRIA): A casualty category
applicable to a hostile casualty, other than the victim of a
terrorist activity, who dies of wounds or other injuries received
in action after having reached a medical treatment facility. Also
called DWRIA. See also casualty category.
Killed in Action (KIA): A casualty category applicable to a
hostile casualty, other than the victim of a terrorist activity,

Class VIII Management
2-10
who is killed outright or who dies as a result of wounds or other
injuries before reaching a medical treatment facility. Also
called KIA. See also casualty category.
NHRC will utilize a robust empirical data base to establish
probability distributions across each of the five sub-categories in
order to develop patient streams. Upon conclusion, NHRC will input
these distributions into modeling systems to generate the materiel
requirements, number and type of supplies, to treat a particular
patient stream. The resulting list of materiel requirements will be
provided to LPC to submit to DLA for future sourcing.
Participants in this Requirements Determination—Surge phase are:
Defense Logistics Agency (Troop Support)
Naval Health Research Center
Deputy Commandant Installations & Logistics (DC I&L) Life Cycle
Management Branch (LPC)
Deputy Commandant Plans, Policies, and Operations (DC PP&O)
Systems in this phase are (See Chapter 9 for Systems Descriptions):
Tactical Medical Logistics Planning Tool (TML+)
a. Process Map
Class VIII Management
2-11
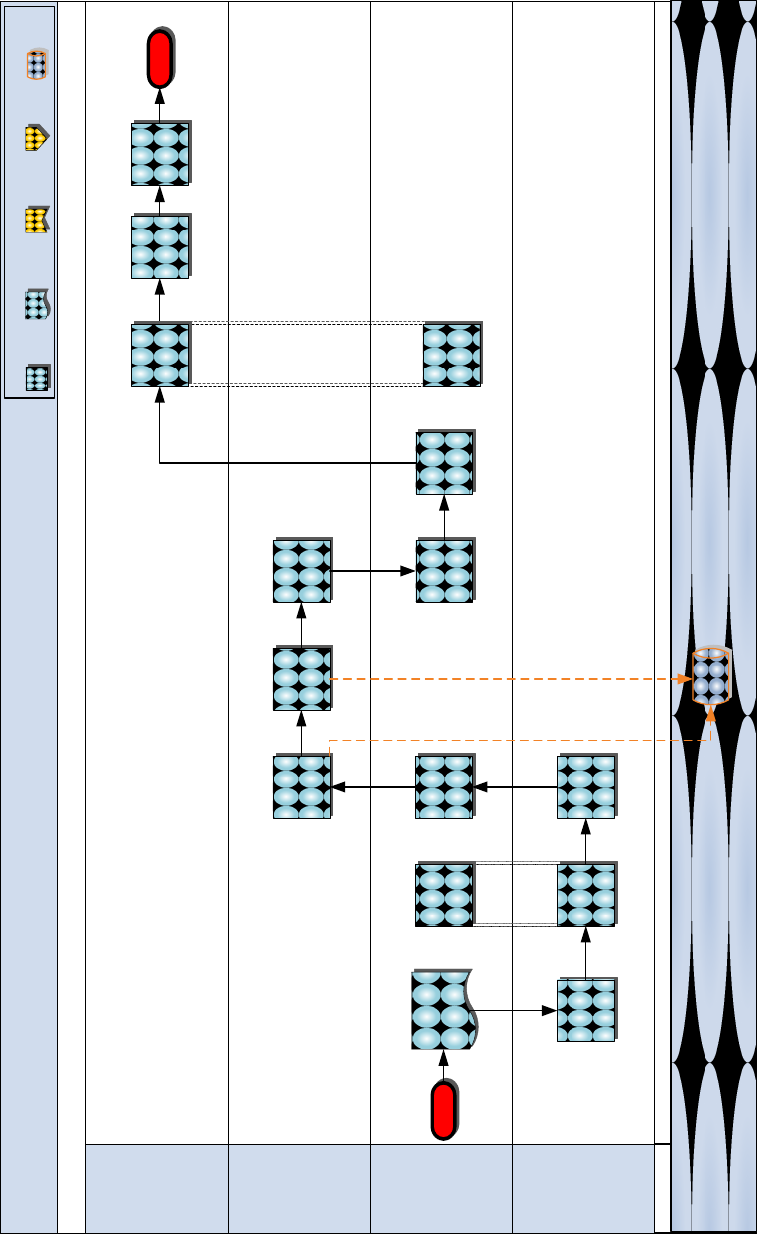
Requirements Determination – Surge (Strategic)
DC PP&O
(PLN)
DLA
(Troop Support)
DC I&LI
(LPC-2)
BUMED
(NHRC)
System
Start
TML+
Release message
to PP&O requesting
CASESTs for
designated
OPLANs
Receive
message and
initiate
coordination
for rate
determination
Coordinate for
determination
of 60/180
DOS
CASESTs
Coordinate for
determination
of 60/180
DOS
CASESTs
Submit 60/
180 DOS
CASESTs
Review &
submit 60/180
DOS
CASESTs
Receive HQMC
CASESTs &
input into
modeling
system
Conduct
modeling
Create HQMC
Medical
Contingency
File (MCF)
Review
USMC surge
requirements
(MCF)
Submit
HQMC MCF
for Biannual
Review
Develop
contract
coverage for
DoD surge
requirements
Host Biannual
review of
medical
contingency
requirements
End
Participate in
biannual
review &
advocate for
USMC
requirements
Publish
DML-PC
DRAFT
Previous
Phase
System
Key:
Next
Phase
Document
Process
Step
Class VIII Management
2-12

Class VIII Management
2-13
b. Process Step Descriptions
1. Release message to PP&O requesting CASESTs for designated OPLANS
Description: Based on annual requirement, DC I&L (LPC-2) create
and disseminate a Naval Message to PP&O (PLN) to request support
in generating CASESTs for designated OPLANS.
Input: Requirements Determination Timeline
Output: CASEST Naval Message
2. Receive message and initiate coordination for rate determination
Description: DC PP&O receive message from DC I&L (LPC-2) and
conduct preliminary analysis required to support the development
of CASEST rates. The preliminary analysis includes an
examination of current Defense and Marine Corps guidance on
considerations for parameter data, as well as planning factors
such as conflict intensities (LIC, MIC, HIC) and DNBI, as defined
above in the introduction to this section.
Input: CASEST Naval Message
Output: Preliminary Analysis
3. Coordinate for determination of 60/180 DOS CASESTs
Description: DC PP&O coordinate with DC I&L (LPO-1/LPC-2) in
order to determine the appropriate CASEST rate for a 60/180 DOS
capability in accordance with the four elements (LIC, MIC, HIC,
and DNBI) defined above in the introduction to this section.
Input: Preliminary Analysis
4. Submit 60/180 DOS CASESTs
Description: DC PP&O (PLN) submit 60/180 DOS CASESTs to DC I&L
(LPC-2) for review.
Output: 60/180 DOS CASESTs
5. Review and submit 60/180 DOS CASESTs
Description: DC I&L (LPC-2) review the CASEST provided by DC
PP&O and make any required adjustments prior to submitting to
NHRC. Upon approval, LPC-2 submit to NHRC.
Input: 60/180 DOS CASESTs
Output: Adjusted 60/180 DOS CASESTs
6. Receive HQMC CASESTs and input into modeling system
Description: BUMED (NHRC) receive HQMC CASESTs and input into
TML+ modeling system.
Input: Adjusted 60/180 DOS CASESTs
Class VIII Management
2-14
7. Conduct modeling
Description: BUMED (NHRC) conduct modeling in TML+. NHRC run the
patient streams in terms of volume and type of injury. Utilize
the model to determine, based on a statistical variables, the
patient stream. Based on this data, determine the associated
AMALs, number of supplies required to treat the patient stream
identified, and generate surge requirements.
Important Note: This process applies to consumable re-supply only
and does not address equipment.
Input: CASEST into TML+
Output: Surge Requirements
8. Create HQMC Medical Contingency File (MCF)
Description: BUMED (NHRC) create HQMC MCF. DC I&L (LPC-2)
participate in this process, along with MCSC (PM CSE), to ensure
that NSNs in the Marine Corps inventory align with the MCF.
Additionally, DC I&L (LPC-2) identify items that are coded ―Y‖
(items that are no longer manufactured but may still be in the
Marine Corps inventory) and determine appropriate substitutions
for replenishment of these items.
Input: Surge Requirements
Output: Draft MCF
9. Review USMC surge requirements (MCF)
Description: BUMED (NHRC) send final MCF to DC I&L (LPC-2) for
review. DC I&L (LPC-2) coordinate with MCSC to ensure all NSNs
associated with the surge requirements are valid. Modify as
appropriate to generate the Adjusted MCF.
Input: Draft MCF
10. Submit HQMC MCF for Biannual Review
Description: DC I&L (LPC-2) submit HQMC portion of the MCF to
DLA (Troop Support) for incorporation with the other service
requirements at the Biannual Review.
Important Note: The MCF is submitted annually in January/July
Output: Final MCF
Class VIII Management
2-15
Figure 8.13 Requirements Determination—Surge (Strategic) Activities
11. Host Biannual Review of medical contingency requirements
Description: Defense Logistics Agency (DLA) (Troop Support) host
Biannual Review of medical contingency requirements. This effort
is supported DC I&L (LPC-2), who participates in the Biannual
Review and advocates for USMC requirements. During the review,
requirements are vetted across all services to ensure
requirements are valid and can be supported by contingency
contracts. A priority is set for items that support multiple
services.
Input: Individual Service MCF
Output: Consolidated MCF
12. Develop contract coverage for DoD surge requirements
Description: DLA review the current contingency contracts in
place to ensure coverage associated with the newly-established
requirements and award contracts for new surge requirements.
Input: Consolidated MCF
13. Publish DML-PC
Description: DLA publish Defense Medical Logistics-Proponent
Committee. The committee involves participants from all
services, who review the status of all contingency files. DML-PC
publish a report at the conclusion of the committee, which covers
the status of contingency files and DLA’s percentage capability.
Output: DML-PC Report

Class VIII Management
3-1
NAVMC 4000.2
Class VIII Management
Selection Criteria
Chapter 3
Class VIII Management
3-3
A. Selection Criteria
Selection Criteria, in conjunction with the Requirements Determination
function described in the following chapter, parallels the Plan Health
Service Support phase of the NAVMEDLOGCOM process for determining
medical support requirements.
The Marine Corps AMALs/ADAL are designed to establish and/or support a
specific health care mission. These health care missions will be
influenced by factors such as fluctuations in combat intensity,
evacuation policies and capabilities, and availability of non-organic
health care support (i.e., Navy Expeditionary Medical Facilities
(EMFs), host nation support, etc). The Marine Corps has established a
process, the Modernization Review, to review the method by which
Medical Materiel is updated to reflect current treatment protocols and
modalities in order to improve operational medical readiness to the
warfighter by fielding improved Medical Materiel.
1. Modernization Review
The Modernization Review is an extensive process and is used to ensure
use of the most-up-to-date equipment and consumables. Individual
AMALs/ADAL are reviewed on a four-year cycle.
Participants in this phase are (See MCO 4400.39, Chapter 8, for
overall roles and responsibilities):
Deputy Commandant Installations & Logistics (DC I&L) Life Cycle
Management Branch (LPC)
Deputy Commandant Combat Development & Integration (DC CD&I)
Integration Division (ID)
Commanding General Marine Corps Systems Command (CG MCSC) Program
Manager, Combat Support Equipment (PM-CSE)
Naval Health and Research Center (NHRC)
Blount Island Command (BIC)
Marine Forces Command (MARFORCOM)
Marine Forces Pacific (MARFORPAC)
Marine Expeditionary Forces (MEFs)
Marine Forces Reserve (MARFORRES)
Systems used in this phase are (See Chapter 9 for Systems
Descriptions):
Medical Logistics Online (MLO)
Total Force Structure Management System (TFSMS)
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management
Class VIII Management
3-4
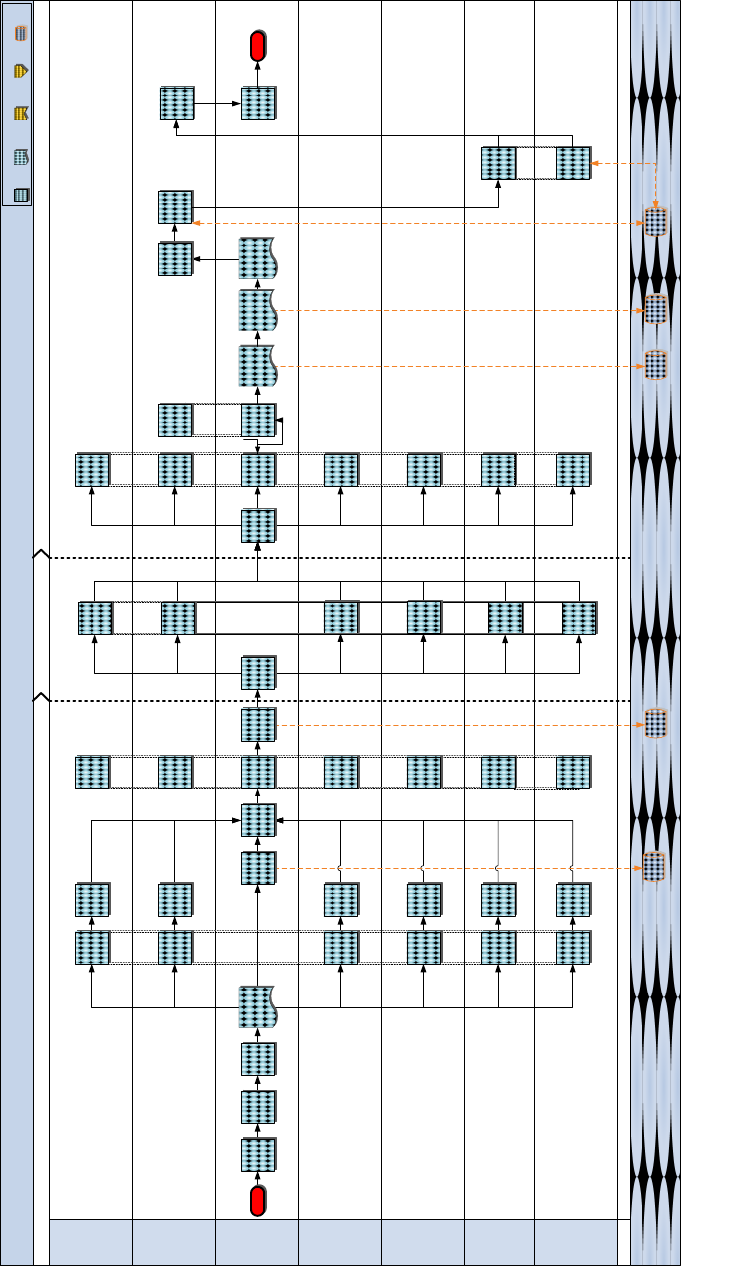
a. Process Map
Figure 8.7 Modernization Review
P o s t - R e v i e w
M od er ni za ti o n Re vi ew
P r e - R e v i e w R e v i e w
D C C D & I
( C D D /L ID / N S B )
S y s t e m
M E F /
M A R F O R R E S
( M L G )
M A R F OR
B I C N H R C
C G M C S C
( P M CS E)
D C I & L
( L P C )
C r e a t e & S u b m i t
A M A L R e v i e w
S u m m a r y R e p o r t
C r e a t e & r e l e a s e
R e v i e w m e s s a g e
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
M L O
P r o v i d e
a t t e n d e e l i s t
D e v e l o p
r e v i e w
s c h e d u l e &
t a i l o r c h e c k l i s t
C o n d u c t P o s t -
R e v i e w
m e e t i n g
C o n d u c t P o s t -
R e v i e w
m e e t i n g
R e c e i v e
A M A L R e v i e w
S u m m a r y
R e p o r t
C o o r d i n a t e
p o s t - R e v i e w
w i t h
d e s i g n a t e d
a t t e n d e e s
C o n d u c t P r e -
R e v i e w
m e e t i n g
P r o v i d e
a t t e n d e e l i s t
C o n d u c t P o s t -
R e v i e w
m e e t i n g
P r o v i d e
a t t e n d e e l i s t
C o m p i l e
R e a d - a h e a d s
a n d d i s t r i b u t e
t o p a r t i c i p a n t s
S y s t e m
R e v i e w 4 y r
A M A L
s c h e d u l e f o r
u p c o m i n g
f i s c a l y e a r
C o o r d i n a t e
P r e - R e v i e w
w i t h
d e s i g n a t e d
a t t e n d e e s
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
C o n d u c t P o s t -
R e v i e w
m e e t i n g
M L O
E n d
L e a d
A M A L s / A D A L
M o d e r n i z a t i o n
R e v i e w
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
P o s t c u r r e n t
l i n e l i s t
M L O
C o n d u c t P r e -
R e v i e w
m e e t i n g
S t a r t
P r o v i d e
a t t e n d e e l i s t
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
M L O
P a r t i c i p a t e i n
A M A L s / A D A L
R e v i e w
P r o v i d e
a t t e n d e e l i s t
C o n d u c t P r e -
R e v i e w
m e e t i n g
P l a n t h e
R e v i e w
P r o v i d e
a t t e n d e e l i s t
C o n d u c t P o s t -
R e v i e w
m e e t i n g
C o n d u c t P o s t -
R e v i e w
m e e t i n g
C o n d u c t P r e -
R e v i e w
m e e t i n g
C o n d u c t P r e -
R e v i e w
m e e t i n g
C o n d u c t P r e -
R e v i e w
m e e t i n g
C o n d u c t P r e -
R e v i e w
m e e t i n g
C o n d u c t P o s t -
R e v i e w
m e e t i n g
R e c e i v e
R e v i e w
M e s s a g e
R e c e i v e
R e v i e w
M e s s a g e
R e c e i v e
R e v i e w
M e s s a g e
R e c e i v e
R e v i e w
M e s s a g e
R e c e i v e
R e v i e w
M e s s a g e
R e c e i v e
R e v i e w
M e s s a g e
C o o r d i n a t e t o
c r e a t e t h e l i n e
l i s t & b u y l i s t
C o o r d i n a t e t o
c r e a t e t h e l i n e
l i s t & b u y l i s t
F i n a l i z e &
d i s s e m i n a t e P o s t -
R e v i e w l i n e l i s t
F i n a l i z e &
d i s s e m i n a t e
M o d e r n i z a t i o n
B u y L i s t
U p d a t e &
S t a f f A M A L
R e v i e w
S u m m a r y
R e p o r t
R e c e i v e t h e
u p d a t e d
S u m m a r y
R e p o r t &
p r o v i d e
f e e d b a c k
R e c e i v e t h e
u p d a t e d
S u m m a r y
R e p o r t &
p r o v i d e
f e e d b a c k
I s s u e
S u m m a r y
L e t t e r & S O N
C r e a t e
F i e l d i n g P l a n
M C A T S
S y s t e mD o c u m e n t
P r o c e s s
S t e p
K e y :
N e x t
P h a s e
P r e v i o u s
P h a s e

Class VIII Management
3-5
b. Process Step Descriptions
1. Review 4 yr AMAL schedule for upcoming fiscal year
Description: MCSC (PM-CSE) review the schedule for upcoming
fiscal year to determine reviews and venues. PM-CSE publish the
schedule via electronic correspondence.
Input: AMAL schedule (4 year)
Output: Schedule for upcoming FY
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx
2. Develop review schedule & tailor checklist
Description: MCSC (PM-CSE) develop review schedule in accordance
with Checklist from AMAL Review Website. PM-CSE tailor Checklist
according to the size and complexity of the AMAL to either a 60-
or 90-Day Post-Review Cycle and post for participant review.
Input: Checklist from AMAL Review Website
Output: Review Schedule, Tailored AMAL Modernization Review
Checklist
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx
3. Plan the Review
Description: MCSC (PM-CSE) plan the review by compiling all
necessary data/information and coordinate logistics at selected
venue. Details pertaining to data/information and the venue for
the review will be incorporated into the Review message.
Input: AAO, SON, NHRC modeling simulation data/information
Output: Details for Review message
4. Create & release Review message
Description: MCSC (PM-CSE) create the Review message,
incorporating details for Review message and staff accordingly.
Upon approval, MCSC (PM-CSE) release the message via electronic
correspondence to the MARFOR; 4
th
MEDLOG; DC CD&I, CDD, LID; NHRC;
BIC; DC I&L; and any other required participants.
Input: Details for Review message
Output: Review Message
5. Receive Review Message
Description: DC I&L, NHRC, BIC, MARFORs, and MEFs/MARFORRES (4
th
MEDLOG) receive the Review message, coordinate internally to
select appropriate staff to support the Review, and prepare
accordingly.
Input: Review Message

Class VIII Management
3-6
6. Provide attendee list
Description: DC I&L, NHRC, BIC, MARFORs, and MEFs/MARFORRES (4
th
MEDLOG) provide MCSC (PM-CSE) a list of personnel designated to
participate in the Review.
Output: Attendee List
7. Post current line list
Description: MCSC (PM-CSE) post current line list to the AMAL
Review workspace for participant evaluation prior to the
Modernization Review.
Output: Current (FY) Line List
8. Coordinate Pre-Review with designated attendees
Description: MCSC (PM-CSE) coordinate with designated personnel
to establish the Pre-Review meeting. The coordination will set
requirements for the meeting (e.g., data/information
requirements, etc.) and determine whether the meeting will be
held in person and/or via teleconference.
Input: Attendee list
9. Conduct Pre-Review meeting
Description: MCSC (PM-CSE) lead designated participants, from DC
I&L, NHRC, BIC, MARFORs, and MEFs/MARFORRES, in a Pre-Review
meeting. The review will address preliminary reference materiel
gathered by PM-CSE to create a consolidated line list, tasker
list, read-ahead documents, etc.
Input: Preliminary Reference Materiel
Output: Consolidated line list, tasker list, read-ahead documents
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx
10. Compile read-aheads and distribute to participants
Description: Upon conclusion of the Pre-Review meeting, MCSC
(PM-CSE) disseminate all documents generated during the Pre-
Review to participants.
Output: Pre-Review: consolidated line list, tasker list, read-
ahead documents
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx, Review
Tab of the corresponding AMAL Review Website
Class VIII Management
3-7
11. Lead/Participate in AMALs/ADAL Modernization Review
Description: MCSC (PM-CSE) lead designated participants,
including DC I&L (LPC), DC CD&I (LID), NHRC, BIC, MARFOR, and
MEF/MARFORRES (MLG), in Modernization Review. The Modernization
Review should include validation of attendee list, presentation
of supporting briefs, and line-by-line reviews. At the conclusion
of the Review, MCSC (PM-CSE) reconcile lists, consolidate notes,
assign preliminary task (task, suspense date, owner), and set
Post-Review Meeting date.
Important Note: The pharmacy is not included in the
Modernization Review. It is the responsibility of the MEDLOGCOs
to procure through the Prime Vendor Pharmacy (PVP) or DLA
Contingency Contracts.
Input: Attendee List, supporting briefs, line list
Output: Reconciled line list, review notes, preliminary task
list, post-review meeting date
12. Coordinate Post-Review with designated attendees
Description: MCSC (PM-CSE) coordinate with designated personnel
to establish the Post-Review meeting and communicates meeting
logistics via e-mail. Additionally, MCSC (PM-CSE) post reconciled
consolidated line list to the Post-Review Tab of the
corresponding AMAL Review Workspace for reference.
Input: Reconciled line list, review notes, preliminary task
list, post-review meeting date
Output: E-mail (Post-Review Coordination)
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx; Review
Tab of the corresponding AMAL Review Website
13. Conduct Post-Review meeting
Description: MCSC (PM-CSE) lead designated participants,
including DC I&L (LPC), DC CD&I (LID), NHRC, BIC, MARFOR,
MEF/MARFORRES (MLG), in Post-Review meeting. Post-Review should
include line-by-line revalidation of information collected at the
Modernization Review and assign action for any outstanding tasks
needed to finalize a proposed line list.
Input: E-mail (Post-Review Coordination)
Output: Revalidated line list, Task List

Class VIII Management
3-8
14. Coordinate to create the line list and buy list
Description: MCSC (PM-CSE) monitor completion of outstanding
tasks in order to create and disseminate Post-Review line list
Once all tasks are complete, DC CD&I (LID, Naval Support Branch)
and MCSC (PM-CSE) refine the output from the Post-Review in order
to ensure alignment with the current SON capabilities and current
missions set by HQMC. Once the line list has been refined, DC
CD&I (LID, Naval Support Branch) and MCSC (PM-CSE) determine the
items required for procurement.
Input: Revalidated line list, Task List
Output: Final line list, Modernization buy list
15. Create and disseminate Post-Review line list
Description: MCSC (PM-CSE) generate the Final Modernization Line
List based on collaboration with DC CD&I (LID, Naval Support
Branch). When completed, MCSC (PM-CSE) upload the Final
Modernization line list to the Post-Review Tab of the AMAL Review
Workspace for reference.
Input: Final line list
Output: Final Modernization line list
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx, Post-
Review Tab of the AMAL Review Website
16. Create and disseminate Modernization Buy List
Description: MCSC (PM-CSE) generate the Final Modernization buy
list based on collaboration with DC CD&I (LID, Naval Support
Branch). When completed, MCSC (PM-CSE) upload the Final
Modernization Buy List to the Post-Review Tab of the AMAL Review
Workspace for reference.
Input: Modernization buy list
Output: Final Modernization buy list
Reference: https://ips.usmc.mil/sites/mefkb/default.aspx, Post-
Review Tab of the AMAL Review Website
17. Create and Submit AMAL Review Summary Report
Description: MCSC (PM-CSE) create and submit AMAL Review Summary
Report to DC CD&I (LID, Naval Support Branch) for review. The
AMAL Review Summary Report will include all changes made to the
line list throughout the review process and the associated
procurement costs.
Input: Final Modernization line list, Final Modernization buy
list
Output: Draft AMAL Review Summary Report
Document: AMAL Review Summary Report

Class VIII Management
3-9
Figure 8.8 Class VIII Modernization Review Activities
Process Descriptions
18. Receive AMAL Review Summary Report
Description: DC CD&I (LID, Naval Support Branch) receive AMAL
Review Summary Report from MCSC (PM-CSE) and review the report to
make any last changes required to ensure the line list and buy
list are in alignment with the SON capabilities and the current
mission set established by HQMC.
Input: Draft AMAL Review Summary Report
19. Update & Staff AMAL Review Summary Report
Description: Upon completing the update to the AMAL Review
Summary Report, DC CD&I (LID, Naval Support Branch) staff the
report to the MARFORs and MEF Surgeons via MCATS for concurrence.
Output: AMAL Review Summary Report
20. Receive the updated Summary Report & provide feedback
Description: MARFORs/MEF Surgeons receive AMAL Review Summary
Report and provide feedback on concurrence/non-concurrence along
with the associated justification to DC CD&I.
Input: AMAL Review Summary Report
Output: Validated AMAL Review Summary Report
21. Issue Summary Letter & SON
Description: DC CD&I (LID, Naval Support Branch) consolidate all
the feedback provided from the MARFORs and MEF Surgeons and issue
a letter to MCSC along with the updated SON, if required, to
MCSC.
Input: Validated AMAL Review Summary Report
Output: Summary Letter, SON (as required)
22. Issue Summary Letter & SON
Description: MCSC (PM-CSE) utilize the issue letter and updated
SON, if provided, to create a fielding plan for procurement and
establish the priorities for the following FY POM-cycle.
Input: Summary Letter, SON (as required)
Output: Fielding Plan
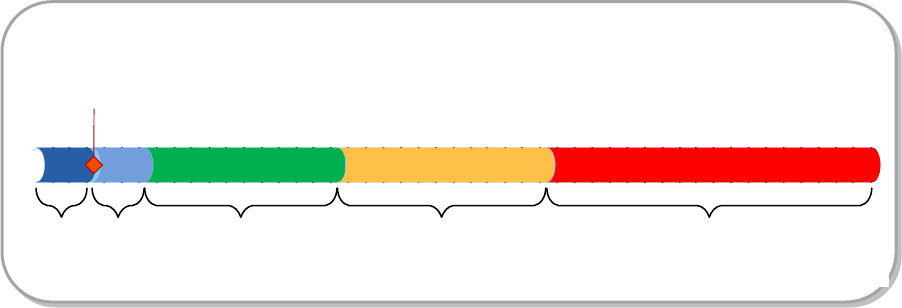
Class VIII Management
3-10
Figure 8.9 Modernization Review Timeline (4yr-cycle)
It is important to note that there are many factors that can impact
the Modernization Review timeline and result in expanding the duration
of each phase.
Major impacts that will halt all forward progress of this timeline are
if funding is not secured or if the requirements fall below the
priority threshold. The priority list is established by DC CD&I and
MCSC based on mission priority. Prioritization fluctuates based on
the funding level received as well as the usage rate.
This phase is crucial to maintaining the timeline. Another example
cause of added time to the review process is if items require research
and development/testing. Therefore, this process is at a minimum 4
years.
2. Fielding and Receiving of Initial Issue
After the Modernization Review is complete, items are purchased in
accordance with the published Modernization Line List and the
Modernization Buy List. MEDLOGCOs can use MLO to view both the buy
list, which displays new items or item increases, and the
modernization list, which shows replacements and new and increased
items.
Once an item is purchased, the item status in MLO will change from
―proposed‖ to ―current.‖ MCSC will then send a spreadsheet list of
items to be received to the MEDLOGCOs in preparation for delivery.
MEDLOGCOs will use the spreadsheet list to generate manual DUE-INs by
line item in DMLSS, which will update when the item is received and
will associate to the appropriate block, thereby creating a Memorandum
of Record receipt (for more information regarding DUE-INs and
Memorandum of Record, please see the ―Assemblage Management (AM)‖
section of the NAVMC 4000.3). Blocks that are out for exercises,
operations, or deployments must be deselected to allow for accurate
updating of the system when those blocks are returned.
Jun - Jan
Field & Evaluate
Jun - Jun
Vendor Coord & Receive Assets
Jul - Jun
Secure Funding
Modernization Review Timeline
Jan - Apr
Pre-Review
Apr - Jul
Post-Review
Apr
Conduct
Modernization
Review
Pre-Review Post-Review
Secure
Funding
Coordinate w/ Vendor &
Receive Assets
Field & Evaluate
3 mo 3 mo 1 yr 1-2 yr 2-3 yr

Class VIII Management
4-1
NAVMC 4000.2
Class VIII Management
Sourcing
Chapter 4
Class VIII Management
4-3
A. Sourcing
The Sourcing function, in conjunction with the Distribution function
described in Chapter 7 (Distribution), parallels the Deliver
Healthcare Capability phase of the NAVMEDLOGCOM process for
determining medical support requirements.
The Class VIII 60 DOS requirement is sourced as follows:
Each MEDLOGCO is required to have a minimum of 15 DOS on-hand.
Any deficiency to the mission requirement is registered with HQMC
and filled through the Global Sourcing Concept. The using unit
will receive the equipment block and associated consumable
blocks, as appropriate, to equal 15 DOS. (See Chapter 10 for the
list of equipment AMALs/ADAL and their associated consumable
blocks).
The surge requirement, consisting of 16-60 DOS are registered
through HQMC portion of the MCF and submitted to DLA (Troop
Support) biannually (See Chapter 3: Requirements Determination
for the detailed process). Requisitions against these pre-planned
requirements are submitted by the MEDLOGCOs to DLA via DMLSS.
DLA (Troop Support) fills the requests through contingency
contracts or traditional support.
If the Combatant Command (COCOM) Theater Lead Agent for Medical
Materiel (TLAMM) or Medical Supply Chain Network is established
in theater in less than 60 days, then the Marine Corps turns over
responsibility of those requirements.
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management

Class VIII Management
4-4
1. Global Sourcing Concept
The Marine Corps uses a Global Sourcing Concept to resource medical
supply deficiencies during a contingency, especially when requirements
exceed current capabilities (AAO). The Marine Corps does not have a
surge capability during a contingency but must utilize the assets
available at the MEDLOGCOs. Therefore in order to prevent a single
MEDLOGCO from becoming non-mission capable in the event of an
OPLAN/CONPLAN deficiency, Class VIII requirements are sent via Naval
Message to HQMC DC I&L (LPO) to coordinate sourcing and direct
redistribution of on-hand AMALs/ADAL.
Participants in the Global Sourcing Concept are (See MCO 4400.39,
Chapter 8, for overall roles and responsibilities):
Deputy Commandant Installations and Logistics (DC I&L) Logistics
Plans and Operations Branch (LPO)
Marine Forces (Pacific/Command)
Marine Expeditionary Forces (MEFs)/Marine Forces Reserve
(MARFORRES), Marine Logistics Group (MLG)
MEF, Medical Logistics Company (MEDLOGCO)
MARFORRES, MLG, 4
th
Medical Logistics Company(MEDLOGCO)*
*4
th
MEDLOGCO in MARFORRES falls under the Medical Logistics Group (MLG) and
supports all MARFORRES units.
Systems used in the Global Sourcing Concept are (See Chapter 9 for
Systems Descriptions):
Defense Medical Logistics Standard Support - Assemblage
Management Module(DMLSS-AM)
Joint Medical Asset Repository (JMAR)
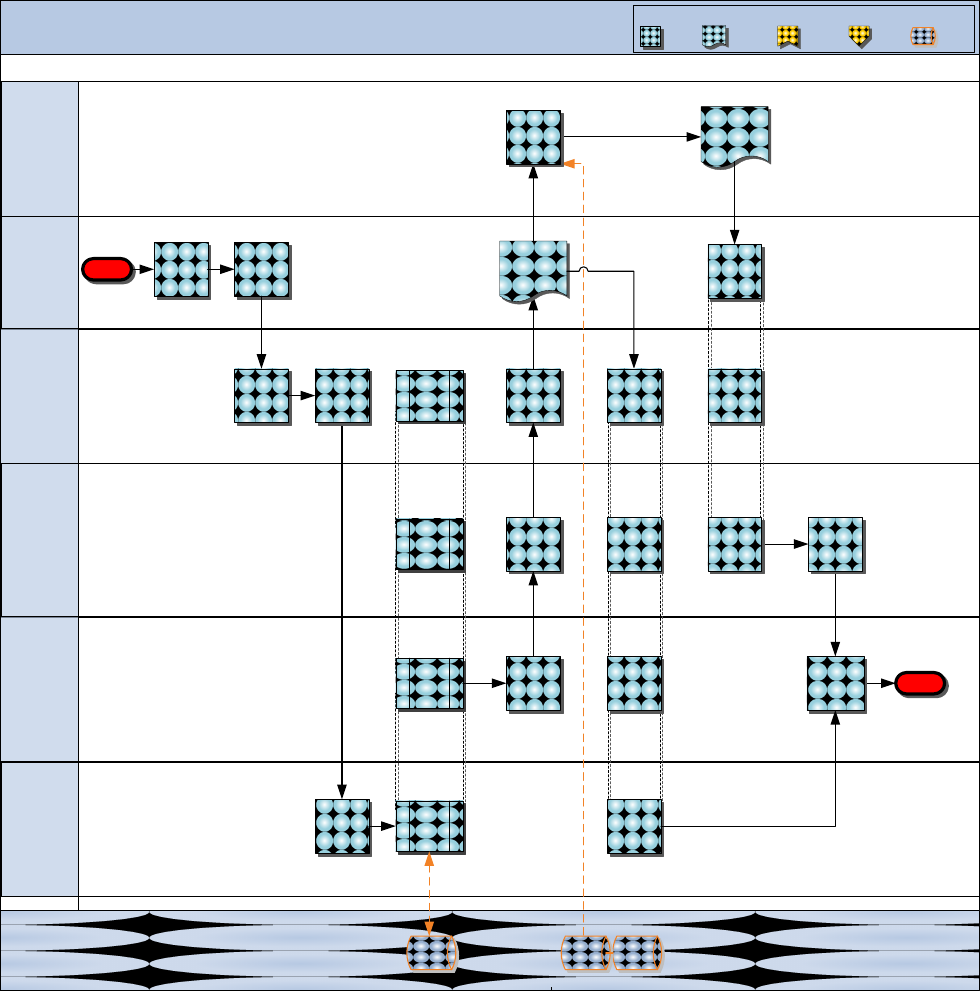
Class VIII Management
4-5
a. Process Map
Figure 8.14 Global Sourcing Process
Global Sourcing
DC I&L
(LPO/LPC)
MSC / MLG
Unit
(Supported)
MARFORMEDLOGCO MEF
System
Start
Determine
mission/task
organization
Receive
Warning/
Execution
Order
Receive
mission/task
organization
Conduct
mission
analysis &
determine
specific
requirements
Designate
Supported
unit
DMLSS-
AM
Endorse
deficiency list
Determine
asset
availability
from other
MEFs
DMLSS-
AM
JMAR
Release message
authorizing the
redistribution of
MEF Class VIII
assets
Review & submit
deficiency list to
HQ for Global
Sourcing
Receive
message
Receive
message
Receive
message
Delegate
coordination
w/
MEDLOGCO
(Supporting)
Coordinate w/
MEDLOGCO
(Supporting)
End
Receive
notification
Receive
notification
Conduct
Pre-LTI
Process
Conduct
Pre-LTI
Process
Conduct
Pre-LTI
Process
Conduct
Pre-LTI
Process
Submit
deficiency list
for global
sourcing
Endorse
deficiency list
Receive
notification
Receive
notification
Document System
Next
Phase
Process
Step
Previous
Phase
Key:

Class VIII Management
4-6
b. Process Step Descriptions
1. Receive Warning/Execution Order
Description: MARFOR receive Warning/Execution Order from HQMC
released by the CJCS. The Execute Order directs execution of an
Operation Order (OPORD) or other military operation to implement
a National Command Authority (NCA) decision. The Warning/Execute
Order will be issued by authority and direction of the Secretary
of Defense (SECDEF) and provides essential guidance such as the
date and time for execution, course of action, major combat
forces approved for the operation, strategic movement planning
guidance, and known logistics constraints. In a particularly
time-sensitive situation requiring an immediate response, an
Execute Order may be issued without prior formal crisis planning.
Input: Warning/Execution Order
2. Determine mission/task organization
Description: MARFOR utilize the guidelines and parameters
established in the Warning/Execution Order to determine the force
structure required to support the particular plan. This force
structure will serve as the basis for the planning of equipment
and personnel required for mission/task execution.
Output: Force List
3. Receive mission/task organization
Description: MEF receive the force list from the MARFOR and use
it to identify unit to support the mission/task. MEF establish
planning guidance for the designated unit.
Input: Force List
Output: Letter of Instruction (LOI)
4. Designate supported unit
Description: MEF provide planning guidance to designated unit to
support the mission/task, in accordance with the Warning/
Execution Order.
Input: Letter of Instruction (LOI)
5. Conduct mission analysis and determine specific requirements
Description: Supported unit review the planning guidance,
conduct internal mission analysis, and determine specific
personnel and equipment/consumable requirements to submit for
approval.
Output: List of Equipment/Consumable Requirements

Class VIII Management
4-7
6. Conduct Pre-LTI Process
Description: Supported unit, MEDLOGCO, MSC/MLG, and MEF conduct
Pre-LTI process (See Chapter 1.2: Pre-LTI process). In this
process, materiel requirements are submitted, validated, and
approved through the Chain of Command. The MEDLOGCO is tasked to
build the AMALs/ADAL in accordance with the MEF’s guidance. The
MEDLOGCO utilize both internal (warehouse) and external (PVM/PVP
and Contingency Contract) to bring the AMALs/ADAL to the desired
level of readiness. In the case that either the desired level of
readiness cannot be achieved through internal/external sourcing
or if completed the MEDLOGCO will become non-mission capable, the
MEDLOGCO creates a Naval Message of Class VIII deficiency
requirements.
Input: List of Equipment/Consumable Requirements
7. Submit deficiency list for global sourcing
Description: MEDLOGCO submit Class VIII deficiency requirements,
which are sent up the Chain of Command for endorsement.
Output: List of Class VIII deficiencies
8. Endorse deficiency list
Description: MSC/MLG and MEF review the Class VIII deficiency
requirements and verify the impact on the mission/task if the
requirements are not filled or if the MEDLOGCO is depleted.
After receiving the MEF’s endorsement, the deficiency list is
submitted to the MARFOR for final review.
Input: List of Class VIII deficiencies
Output: List of Class VIII deficiencies(Endorsed)
9. Review & submit deficiency list to HQ for Global Sourcing
Description: MARFOR review the deficiency list and the risk
associated with not filling the requirement and approve it. Upon
approval, MARFOR create a Global Sourcing Naval Message and
submits to HQMC DC I&L (LPO) to coordinate sourcing and direct
redistribution of on-hand AMALs/ADAL.
Input: List of Class VIII deficiencies (Endorsed)
Output: Global Sourcing Naval Message
10. Receive notification
Description: MSC/MLG, MEDLOGCO, and supporting unit receive a
courtesy copy of the Global Sourcing Naval Message and prepare
for any required task to complete the process.
Input: Global Sourcing Naval Message
11. Determine asset availability from other MEFs
Class VIII Management
4-8
Figure 8.15 Global Sourcing Activities Process Descriptions
Description: DC I&L (LPO/LPC) review the deficiency request
provided in the Naval Message and determine the readiness status
of the other MEDLOGCOs in JMAR. LPO/LPC determine the best source
internal to the Marine Corps for the assets required associated
with the least amount of risk.
Important Note: JMAR is populated by DMLSS.
Input: Global Sourcing Naval Message
Reference: ―Asset Review‖ section in NAVMC 4000.3.
12. Release message authorizing the redistribution of MEF Class VIII
assets
Description: DC I&L (LPO/LPC) generate a Naval Message
designating the supporting MEDLOGCO and the quantity and type of
assets to provide to the requesting unit as well as any
amplifying guidance.
Output: Class VIII Redistribution Naval Message
13. Receive message
Description: MARFOR, MEF, and MSC/MLG receive the Class VIII
Redistribution Naval Message and complete respective tasks in
accordance with HQMC guidance.
Input: Class VIII Redistribution Naval Message
14. Delegate coordination w/ MEDLOGCO (Supporting)
Description: MSC/MLG task the MEDLOGCO to coordinate with the
designated MEDLOGCO as defined in the guidance message to arrange
for inventory and transportation of assets.
Output: Global Sourcing Task
15. Coordinate w/MEDLOGCO (Supporting)
Description: MEDLOGCO coordinate with MSC/MLG and the Supported
unit to support the conclusion of the LTI with the supporting
MEDLOGCO and to arrange for transportation of the asset to a
location set by the supported unit. The coordination is complete
when the asset is received by the supported unit.
Input: Global Sourcing Task
Output: Requested AMALs/ADAL
Class VIII Management
4-9
2. Sourcing Timeline
Since the MEDLOGCOs are PVM/PVP customers, it is assumed that the
MEDLOGCO will utilize the PVM/PVP prior to submitting any Class VIII
requisitions to DLA. It is important to note that PVM/PVP support
both usage and non-usage base items. Usage items are frequently used
materiel for which previsions are built into the contract for a
guaranteed fill rate the PVM/PVP is responsible to meet. Non-Demand
items are materiel that the PVM/PVP may or may not have in stock since
the demand is low or infrequent, and therefore, the PVM/PVP cannot
guarantee fill rates. The Marine Corps falls into the non-demand
side, so there is no guarantee that the PVM/PVP will be able to
fulfill the requests. When the PVM/PVP cannot fulfill a request, the
Marine Corps will receive a canceled or rejected line in DMLSS. At
this point, the MEDLOGCO can submit a requisition to DLA (Troop
Support). For more information on non-demand fill rates and the
provisions for days to fill non-demand items, visit DMMOnline,
https://dmmonline.dscp.dla.mil. Additionally, please see the
―Resubmit Orders/Follow-Up Requests/Cancellations‖ section of the
NAVMC 4000.3, for more information regarding cancellation/rejection
lines in DMLSS.
The MEDLOGCO submits a requisition to DLA’s source of supply in DMLSS.
There are two means by which DLA (Troop Support) sources materiel by
contingency contracts and traditional support.
When a requisition is submitted, it is first received by DLA
(Transaction Services). DLA (Troop Support) will pull the requisition
associated with any readiness customer and attempt to route the
requirement to an appropriate contingency contract, if available.
Since contingency contracts guaranteed access to the materiel when
needed, DLA only has to coordinate the shipping of materiel and can
deliver the asset within 72 hours of the request. For more
information on the materiel covered by current contingency contracts,
visit DMMOnline, Readiness Management Application at
https://dmmonline.dscp.dla.mil.
Requests that are not covered by contingency contracts are routed
through DLA’s Enterprise Business System (EBS). This is referred to
as traditional support in which DLA will coordinate with a buyer to
procure the requested item. Since this process is influenced by
procurement and acquisition lead times, it may take months to deliver
to the requesting unit.
(See Chapter 6: Acquisition for information about the process for
funding of requisitions, contingency contracts, and traditional
support.)
Class VIII Management
4-10
Figure 8.16 Class VIII Sourcing Timeline
During a contingency, when the Marine Expeditionary Brigade (MEB) is
employed, the Class VIII assets held aboard the Maritime
Prepositioning Ship Squadron (MPSRON) are available for employment
consideration. However, if a MEB is not employed, assets must be
requested from the owning Service, the COCOM, and the Joint Chiefs of
Staff (JCS). Approval from all three levels is required for the
release of Class VIII assets.
Once the TLAMM is established in a theater of operations, the TLAMM
assumes responsibility of Class VIII resupply. The TLAMM is a joint
initiative established in a theater of operations under the
responsibility of the Combatant Commander. (Note: The TLAMM typically
is the Army.)
Days of Supply
6 5
JunApr
150 60 15180
Apr - Apr
Source of
Materiel
Jun - OctApr - Jun
Requirement
4/6/2010 - 6/5/2010
MEDLOGCO/
Prime
Vendor
DLA (Troop Support)
DLA (Troop Support) or TLAMM
(Ad Hoc Requirements)
USMC Global Sourcing
On-hand Surge Sustainment

Class VIII Management
5-1
NAVMC 4000.2
Class VIII Management
Positioning
Chapter 5
Class VIII Management
5-3
A. Positioning
The Positioning function parallels the Sustain Healthcare Capability
phase of the NAVMEDLOGCOM process for determining medical support
requirements.
Positioning of Class VIII materiel, as defined by MCO 4400.39, does
not occur since there is no stockpile of Class VIII materiel beyond
the stocks held by the MEDLOGCOs and the stocks held aboard MPF (See
HQMC Prepositioning Programs Handbook, 2
nd
Edition for details
pertaining to management of MPF stocks). DLA (Troop Support) utilizes
contingency contracts with PVM/PVP, which pull materiel from warehouse
locations on an as-needed basis to fill requirements during days 16-
60. After day 60, materiel is positioned by DLA (Troop Support) in the
theater of operations with the COCOM TLAMM or Medical Supply Chain
Network.
1. Positioning of Class VIII at Blount Island Command (BIC)
All Class VIII equipment and consumables required to support an MPF
for thirty days of contingency operations will be prepositioned aboard
MPF ships. Class VIII supplies and equipment on MPSRON ships are
intended to fill the gap between days 16-30. The following items will
not be embarked due to shelf-life and other issues:
Narcotics/controlled substances
Precious metals
Refrigerated Class VIII items (refrigerated items will not be
embarked aboard MPF vessels until adequate container space is
identified)
All Federal Supply Class 6505 materiel, except for intravenous
(IV)
Solutions, medical gasses, and pharmaceuticals that are part of
the Capability Sets (CAPSET)
Federal Supply Class Non-6505 (all others) with remaining shelf
lives of 36 months or less (exception managed)
Items that do not meet prepositioning criteria will be included in the
Fly-In-Echelon (FIE). It is the responsibility of the supporting MEF
to purchase and ship all reported FIE requirements. Blount Island
Command (BIC) will provide After Action Report (AAR) post-loading of
an individual MPS to the supporting MEF. The report will identify all
FIE, not-in-stock, and short shelf life requirements. The respective
MEF/MEDLOGCO will have ready stock or utilize DLA contingency
contracts to support FIE requirements.
2. Geo-locations of Class VIII Materiel Positions
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management

Class VIII Management
5-4
Figure 5.1 Class VIII Materiel Positioning Geo-locations
MCPP-N
MFR
BIC
3
rd
MEDLOGCO
1
st
MEDLOGCO
2
nd
MEDLOGCO

Class VIII Management
6-1
NAVMC 4000.2
Class VIII Management
Acquisition
Chapter 6
Class VIII Management
6-3
A. Acquisition
The Acquisition function parallels the Acquire Healthcare Enablers
phase of the NAVMEDLOGCOM process for determining medical support
requirements.
The MEF commander is responsible for the funding, requisitioning,
maintenance, and management control of all Class VIII materiel
allowance items in his possession with Operation and Maintenance,
Marine Corps (O&MMC) funds. (See NavCompt Manual, Volume 7, paragraph
075001-25 for additional funding guidance.)
MARFORRES is responsible for submitting funding requests in form of a
MARFORRES Training, Exercise and Employment Plan Database (TEEP)
annually to HQMC. The TEEP is generated from a consolidation of
MARFORRES exercises and anticipated training costs for the upcoming
FY.
MCSC is responsible for the procurement of Initial Issue (15 DOS) and
Modernization of Class VIII through the Warfighting PEB.
The Warstopper Program is a Defense O&M line of funding that is used
to support contingency contracts. There are various lines of funding
within the Warstopper program, one of them is the medical readiness
line. This line funds the fees to the vendors to maintain the
availability of the materiel to which they are required to have
access. The Warstopper Program enables DLA to bypass the
administrative lead times and procurement lead times, required for
traditional acquisition, and guarantees access. It is important to
note that the Warstopper Program is not for the purchase of materiel
or equipment, but rather the guaranteed access to the materiel when
needed.
The Marine Corps does not incur any cost for Contingency Contracts
until the requisitions are submitted. The MEDLOGCOs are responsible
for ensuring the funds have been obligated in SABRS prior to
submitting a requisition. Subsequently, the MEDLOGCO submits a
requisition in DMLSS with a DODAAC and a funding code. In the case of
a contingency operation, the funding code is provided in the Execution
Authorization Message (See Chapter 7: Distribution: Execution
Authorization Message Activities). DLA charges the account through
DFAS. On the backside, the Defense Finance and Accounting System
(DFAS) will then allocate funds to the vendor upon receipt of an
invoice.
The Defense Working Capital Fund is used to support traditional
acquisition for the purchase of materiel or equipment. Traditional
support is required for assets that are specific to the military or
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management
Class VIII Management
6-4
are non-demand items for which a commercial vendor cannot provide
guaranteed access.

Class VIII Management
7-1
NAVMC 4000.2
Class VIII Management
Distribution
Chapter 7
Class VIII Management
7-3
A. Distribution
The Distribution function, in conjunction with the Sourcing function
described in Chapter 4 (Sourcing), parallels the Deliver Healthcare
Capability phase of the NAVMEDLOGCOM process for determining medical
support requirements.
The Medical Air Bridge (MAB) is the means by which DLA (Troop Support)
distributes Class VIII surge requirements (See Chapter 3: Requirements
Determination for more information on how surge requirements are
submitted). The MAB provides materiel to an Aerial Port of
Debarkation (APOD) within 72 hours of the request. Each MEDLOGCO is
an authorized MAB user. Upon receiving a requisition, DLA will
determine location of another authorized user in closest proximity to
a USTRANSCOM carrier. The materiel is consolidated and turned over to
the USTRANSCOM authorized carrier and then flown to an APOD. DLA is
authorized to utilize MAB locations in conjunction with USTRANSCOM
carriers; this precludes the requirement to send the materiel to a
Consolidation and Containerization Point (CCP) at one of the Defense
Distribution Centers and thereby decrease the lead time.
Constraints to the MAB that will increase the delivery time of
materiel are the shipment of non-air authorized HAZMAT, Class II
narcotics, and bottled gas. More information on materiel constraints
and restrictions can be found at DMMOnline,
https://dmmonline.dscp.dla.mil.
Asset visibility for the MAB is maintained though standard commercial
marking or government Global Tracking System (GTS). In the case of
commercial marking, DLA uses the preferred marking method of the
authorized USTRANSCOM carrier. Regardless of which carrier is used,
the Medical Air Bridge database will depict the tracking number for
all assets in-transit, available to all authorized users.
Government GTS is used if the asset is delivered to a government
airbase. In this case, all assets will be marked with Radio Frequency
Identification (RFID) tags.
Additionally, the Emergency Supply Operation Center (ESOC) is the
DLA’s resource for transferring materiel to units in an emergency
within 24 hours. In garrison, units in possession of a Department of
Defense Activity Address Code (DODAAC), a Prime Vendor account, and
access to the internet can order directly from PVM/PVP. If PVM/PVP is
unable to provide the materiel, DLA can order the materiel directly.
Units can also call the distribution center in order to arrange a
special pickup of materiel if an emergency situation arises.* Units
that are deployed must contact the TLAMM in their Area of
Selection
Criteria
Requirements
Determination
Sourcing Positioning Acquisition Distribution
Management

Class VIII Management
7-4
Responsibility (AOR), establish an account and a line of accounting
(LOA), and place their order through the TLAMM. The TLAMM will
establish and arrange pickup and transportation of the materiel to the
unit’s location.
*DLA’s ESOC can be contacted at 215-737-2112.
1. MEDLOGCO Ordering/Receiving Process in a Forward Area
Prior to deployment, MEDLOGCO should review the Medical Contingency
File (MCF) and determine the actual line items and quantities that
will be required for the specific mission. The MEDLOGCO may want to
utilize NHRC and/or TML+ to determine the appropriate re-supply based
on mission criteria (size, scope, location, anticipated CASEST). The
MCF should be loaded in DMLSS as a separate ―assemblage‖ with all NSN
pointing to SMS as the source of supply. With the approval of
MEF/SUPBN, MEDLOGCO should validate requirements and quantity,
determine the delivery date and/or APOD/APOE, and execute the MCF
electronic order. It is recommended that MEDLOGCO coordinate orders
and establish timelines with DLA TS prior to submission in order to
ensure the use of available contingency contracts within DLA. This
inventory will provide the foundation inventory for the MEDLOGCO to
establish a forward deployed warehouse for line item re-supply of the
deployed AMALs/ADAL.
Once deployed, the MEDLOGCO will be the USMC lead agent for medical
supplies and consumables. All guidelines and regulations regarding the
ordering and receiving of Class VIII will be established through the
MEDLOGO in accordance with the TLAMM. The following steps provide a
general overview of the ordering and receiving process for Class VIII
materiel while units are in a forward area:
Upon arrival in the forward area, using units must register
with the MEDLOGCO and submit a Delegation of Authority (DOA)
for Narcotics and identified LNO’s
Orders will be placed via the Point of Contact (POC) to
MEDLOGCO customer service
Orders will be processed by MEDLOGCO and ordered
Once materiel has been receipted, MEDLOGCO will notify the
using units via email and phone
After orders have been received, MEDLOGCO will ship
materials to the unit by the most expedient mode of
transportation; all material will be tagged by a tracking
device to ensure that the shipment is accounted for until it
reaches its destination
Please note that this process may vary according to situation or
events on the ground.

Class VIII Management
8-1
NAVMC 4000.2
Class VIII Management
Marine Forces Reserve
(MARFORRES)
Chapter 8
Class VIII Management
8-3
A. Marine Forces Reserve (MARFORRES)
As the Reserve Component (RC) of the Marine Corps Total Force, Marine
Forces Reserve (MARFORRES) reinforces and augments the Active
Component (AC) either by integration of RC forces with AC Marine Air
Ground Task Forces (MAGTFs), and/or through the provision of
capabilities to directly satisfy Combatant Commander requirements. As
an Operational Reserve, MARFORRES must possess sufficient Class VIII
materiel to support inactive duty and annual training, as well as
short-duration deployments at the Regiment/Group level and below.
When integrating with AC MAGTFs, MARFORRES units will obtain medical
support and sustainment from the gaining AC command’s medical
infrastructure, including the MEDLOGCO that supports that AC command.
4
th
Marine Logistics Group (MLG), 4
th
Supply Battalion, 4
th
Medical
Logistics Company will be the custodians for all medical supplies and
equipment for MARFORRES. Initial requirements for AMALs/ADAL support
will be forecasted through the USMC force allocation and MARFORRES
Training and Exercise Employment Plan (TEEP) processes. All AMALs/ADAL
will be ordered from 4
th
MEDLOGCO 60 days from the date of exercise via
a Request for Additional Medical Materiel Letter (See MARFORRES Force
Order 6000, Chapter 7 for a sample of the letter). All requests will
be sent to MARFORRES G4 Health Service Support Officer (HSSO) and
routed through the Chain Of Command. Units requiring support from 4
th
MEDLOGCO will submit their billable line of accounting in their
request for support, which will be billed at the beginning of the
operation. JLTIs will be conducted Pre- and Post-exercise in
accordance with Force Order 6000.1B Chapter 7. Once the equipment is
signed for, coordination of movement of AMALs/ADAL to the exercise and
post-exercise will be accomplished by the using unit. Subsequently,
all AMALs/ADAL will be returned to 4
th
MEDLOGCO in the same
configuration that they were inspected. All other instruction for
Class VIII materiel can be found in Marine Forces Reserve Force Order
6000.1B.
Per Force Order 6000, 4
th
Medical Logistics Company does not maintain
stocks of controlled substances. These stocks must be requested from
an AC Medical Logistics Company of a MTF by a Medical Officer
supporting the exercise or AT.

Class VIII Management
9-1
NAVMC 4000.2
Class VIII Management
Systems Descriptions
Chapter 9
Class VIII Management
9-3
A. Systems Descriptions
Acronym
Name
Description
Reference
DMLSS
Defense Medical
Logistics
Standard Support
Delivers an automated and integrated
information system with a comprehensive range
of medical materiel, equipment, war reserve
materiel and facilities management functions
for the Military Health System. Of note, DMLSS
provides line item control only.
NAVMC
4000.3/eLearning
access:
https://jml149.dmlss
.detrick.army.mil/DM
LSSU/
GCSS-MC
Global Combat
Support System-
Marine Corps
A portfolio of systems that supports the
logistics elements of Command and Control,
joint logistics interoperability, and secure
access to and visibility of logistics data.
The GCSS-MC portfolio includes LDW, Log C2,
and several legacy systems, and portfolio
requirements are contained in the GCSS-MC ORD.
Of note, GCSS-MC also includes SASSY.
http://www.marcorsys
com.usmc.mil/sites/g
css-mc/index.aspx
JMAR
Joint Medical
Asset Repository
The Quad-Service web based medical logistics
data warehouse and data repository that serves
as a single source for acquiring, managing,
and providing timely and accurate joint
medical asset visibility information. JMAR
collects medical logistics data from 31 DoD
sources systems and makes that data available
through standard and customized reports.
https://jmar.detrick
.army.mil
MLO
Medical Logistics
Online
Medical Logistics Online is a database that
helps in keeping a repository of Marine Corps
medical logistics statistics and information.
https://ips.usmc.mil
/sites/mefkb/default
.aspx
PBDD
Program Budgeting
Documentation
Database
The PBDD, a web-based system accessible to all
Marine Corps users, is the Corps' primary means
of gathering data for program development. It
facilitates controlled, orderly, timely
staffing and editing of initiatives by program
sponsors, advocates, and HQMC departments.
PBDD collects and presents data for POM
deliberations and will facilitate the
collection and display of the standardized
performance measures and cost data.
https://hqipom1.hqmc
.usmc.mil/portal/ser
vlet/GlobalLogin
SABRS
Standard
Accounting
Budgeting And
Reporting System
The official accounting system for the U.S.
Marine Corps, and was designed to meet all
fiduciary standards established by the General
Accounting Office. This includes the year
authorized to incur new obligations and the
subsequent five year to complete receipt and
expenditures on established undelivered
orders.
MCO P7300.12
SASSY
Supported
Activities Supply
System
The Automated Information System (AIS) that
supports retail level of U.S. Marine Corps
supply system. SASSY provides the retail
supply accounting functions, such as stock
replenishment, requirements determination,
receipt inventory, stock control, and asset
availability for all U.S. Marine Corps units.
Of note, SASSY provides TAMCN control only.
UM4000.124 SASSY
User Manual
TFSMS
Total Force
Structure
Management System
TFSMS is a Marine Corps (MC) enterprise system
integrating capability development processes
to support the Warfighter in terms of
structure and equipment.
https://tfsms.mccdc.
usmc.mil

Class VIII Management
9-4
Acronym
Name
Description
Reference
TML+
Tactical Medical
Logistics
Planning Tool
TML+ is used in calculating the casualties for
any given operation, as well as patient flow.
The output from this system is used to
determine the materiel requirements to meet
the need of planned operations. TML+ also
helps in identifying the quantity of supply
and personnel, and transportation assets
needed for support.
TML+, Tactical
Medial Logistics
Planning Tool User’s
Manual Version 4.1
Figure 8.17 Systems Associated with Class VIII

Class VIII Management
10-1
NAVMC 4000.2
Class VIII Management
AMALs/ADAL Set List
Chapter 10
Class VIII Management
10-3
A. AMALs/ADAL Set List
Figure 8.18 AMALs/ADAL Set List
AMAL TAMCN
DESCRIPTION NSN IDNO CONCEPT OF EMPLOYMENT
Equipment /
Consumable Ratio
618 C86008
LABORATORY EQUIPMENT 6515090006459 11712A 1 Labs; Laboratory testing for surgical casualties and DNBI
619 C86048
LABORATORY CONSUMABLES 6515090006455 11706A 100 Pts: hematology, chemistry, urinalysis, blood bank, microbiology
627 C86148
X-RAY EQUIPMENT 6515090006454 11707A 1 X-ray Suites; rapid electronic imaging system
631 C86248
SHOCK SURGICAL TRIAGE EQUIPMENT 6515090006496 11740A 1 STP Sites
632 C86288
SHOCK SURGICAL TRIAGE CONSUMABLES 6515090006494 11736A 50 Trauma Cases
633 C86308
ACUTE CARE WARD EQUIPMENT 6515090006475 11725A 10 Flow Through Beds (6 cot/bed and 4 Critical care)
634 C86348
ACUTE CARE WARD CONSUMABLES 6515090006476 11726A 50 Bed Days (72hr/pt)
635 C86388
BATTALION AID STATION EQUIPMENT 6515090006473 11723A Tx 300 DNBI Pts / 30 days
636 C86408
BATTALION AID STATION CONSUMABLES 6515090006472 11722A Tx 300 DNBI Pts / 30 days
637 C86448
PREVENTIVE MEDICINE MANEUVER (PMM) EQ 6515090006495 11735A
Inspect food service, sanitation , water potability, and disease
surveillance
638 C86488
PREVENTIVE MEDICINE TECHNICIAN (PMT) Con 6515090006497 11741A
Inspect food service, sanitation , water potability, disease surveillance
and control, habitability & w aste management
639 C86508
OPERATING ROOM EQUIPMENT 6515090006474 11724A 1 OR’s / 2 Tables
640 C86548
OPERATING ROOM CONSUMABLES 6515090006479 11731A 25 Cases
645 C87458
FORWARD RESUSCITATIVE SURGERY SYSTEM 6515090002014 10882A 18 Pts for 48 hours per every 646 re-supply
646 C86568
FRSS RESUPPLY 6515090006501 18 Pts for 48 hours
647 C87008
EN ROUTE CARE SYSTEM (ERCS) 6515090006483 11729A
Monitor/transport (2) critically injured but stabilized for tw o hour; CH46,
CH53, MV22
648 C80048
SHORT RANGE CASUALTY EVACUATION
(CASEVAC)
6515090006457 11710A
Monitor/transport (5) litter casualties and (2) ambulatory for tw o hours
650 C80068
PREVENTIVE MEDICINE OCCUPATIONAL
SURVEILLANCE
6515090006478 11728A
Conduct industrial hygiene functionality and env health assays
651 C80078
PREVENTIVE MEDICINE ENTOMOLOGY (PM ENTO) 6515090006486 11730A conduct entomology functionality
652 C80058
CHEMICAL BIOLOGICAL INCIDENT RESPONSE
FORCE
6515090006456 11709A
Casualty collection, non-ambulatory decon and Pt Holding/stabilization
660 C80098
MARINE CORPS SPECIAL OPERATIONS
COMMAND
6515090006458 11711A
Provide initial resuscitative and stabilizing care for 14 pt with major
injuries
662 C87158
FIELD DENTAL OPERATORY 6515090001996 10880A TX 200 Dental Pt / 30 days
685 C86858
GEO MISSION / COLD WEATHER 6515090006498 11742A 1000 PAR / 30 days; Diagn & Tx for Freezing and non-freezing injuries
686 C86868
GEO MISSION / HOT WEATHER 6515090006500 11739A
1000 PAR / 30 days; prophylaxis & Tx diseases endemic to hot
w eather
687 C86878
GEO MISSION/NBC PER INDIVIDUALS 6515090006499 11738A
Provides self-administered medicants for a singel CBRN exposure,
1 per T/O
688 C86888
GEO MISSION/NBC UNIT 6515090006485 11734A 1000 PAR / 30 days; Augmnet BAS/Sickcall in tx of CBRN casualties
691 C86948
MEDLOG TEST/REPAIR EQUIPMENT 6515090006480 11733A
Perform testing, calibrating and level three repair support for Class VIII
equipment
692 C86988
MEDLOG TEST/REPAIR CONSUMABLES 6515090006477 11727A
Perform testing, calibrating and level three repair support for Class VIII
equipment
699 C87408
SICK CALL BLOCK 6515090006484 11732A 300 DNBI PT / 30 days
JBAIDS C80008
JOINT BIOLOGICAL AGENT IDENTIFICATION AND
DIAGNOSIS SYSTEM (JBAIDS)
6545015292063 11288A
Biological agent identification and diagnostic system
(1) 691: (1) 692
USMC AMAL/ADAL
(1) 618: (2) 619
(1) 631: (2) 632
(1) 633: (2) 634
(1) 635: (2) 636
(1) 639: (2) 640
(1) 645: (5) 646
USMC Medical Kits
N/A C31502F
INDIVIDUAL FIRST AID KIT (IFAK) 6545015392732 04351D
Self-aid for life threatening external bleeding and common non-life
threatening injuries
N/A C60088
COMBAT LIFE SAVER (CLS) KIT 6545015714470 11705A
Tx extremity hemorrhage, tension phuemothrorax and airw ay
management; 1st responder care at point of injury by CLS trained
Marines
N/A C00262B
STANDARDIZED VEHICLE MEDICAL KITS 6545015722054 11717A 1st responder care at point of injury

Class VIII Management
11-1
NAVMC 4000.2
Class VIII Management
Terms of Reference
Chapter 11
Class VIII Management
11-3
A. Acronyms & Definitions
1. Acronym List
AAO
Approved Acquisition Objective
ADAL
Authorized Dental Allowance List
ALCM
Assemblage Life Cycle Management
AMAL
Authorized Medical Allowance List
APOD
Aerial Point of Debarkation
BIC
Blount Island Command
BMT
Bio-Medical Technicians
BUMED
Bureau of Medicine and Surgery (United States Navy)
CASEST
Casualty Estimation
CBRN
Chemical, Biological, Radiological, Nuclear
CCP
Consolidation and Container Point
CDD
Combat Development Directorate
CG
Commanding General
CLB
Combat Logistics Battalion
COCOM
Combatant Commander
CONPLAN
Contingency Plan
DC CD&I
Deputy Commandant for Combat Development and Integration
DC I&L
Deputy Commandant for Installations and Logistics
DC PP&O
Deputy Commandant for Plans, Policies, and Operations
DLA
Defense Logistics Agency
DMLSS
Defense Medical Logistics Standard Support
DMLSS-AM
Defense Medical Logistics Standard Support-Assemblage
Management Module
DMLSS-AIS
Defense Medical Logistics Standard Support-Automated
Information System
DML-PC
Defense Medical Logistics-Proponent Committee
DMMPO
Defense Medical Management Program Office
DNBD
Disease Non-Battle Death
DNBI
Disease Non-Battle Injury
DOD
Department of Defense
DODAAC
Department of Defense Activity Address Code
DOS
Days of Supply
DWRIA
Died of Wounds Received in Action
EBS
Enterprise Business System
EFDS
Expeditionary Force Development System
EMF
Expeditionary Medical Facility
FY
Fiscal Year
GCSS-MC
Global Combat Support System-Marine Corps
GFC
Gaining Force Commander
GTS
Global Tracking System
HAZMAT
Hazardous Material
HIC
High Intensity Conflict
HSSE
Health Services Support Element
HSSO
Health Services Support Officer
HQMC
Headquarters, Marine Corps
ID
Integration Division
JCIDS
Joint Capabilities Integration Development System
JCS
Joint Chiefs of Staff
Class VIII Management
11-4
JLTI
Joint Limited Technical Inspection
JMAR
Joint Medical Asset Repository
KIA
Killed in Action
LCE
Logistics Combat Element
LIC
Low Intensity Conflict
LID
Logistics Integration Division
LOA
Line of Accounting
LOI
Letter of Instruction
LPC
Life Cycle Management Branch
LPO
Logistics Plans and Operations Branch
LTI
Limited Technical Inspection
MAB
Medical Air Bridge
MAGTF
Marine Air Ground Task Force
MARADMIN
Marine Administrative Message
MARCORSYSCOM
Marine Corps Systems Command
MARFOR
Marine Force
MARFORCOM
Marine Forces Command
MARFORRES
Marine Forces Reserve
MCATS
Marine Corps Action System
MCF
Medical Contingency File
MCWL
Marine Corps Warfighting Lab
MEDLOG
Medical Logistics
MEDLOGCO
Medical Logistics Company
MEU
Marine Expeditionary Unit
MIC
Medium Intensity Conflict
MLO
Medical Logistics Online
MPS
Maritime Prepositioning Ships
MPSRON
Maritime Prepositioning Squadron
NAVCOMPT
Navy Comptroller
NCA
National Command Authority
NHRC
Naval Health Research Center
NTTP
Navy Tactics, Techniques, and Procedures
OPORD
Operations Order
PBDD
Program Budgeting Documentation Database
PM-CSE
Program Manager-Combat Support Equipment
PP&O
Policy Plans and Operations
PVM
Prime Vendor Medical Surge
PVP
Prime Vendor Pharmacy
QA/QC
Quality Assurance/ Quality Control
RFID
Radio Frequency Identification
RO
Responsible Officer
SABRS
Standard Accounting Budgeting and Reporting System
SASSY
Supported Activities Supply System
SECDEF
Secretary of Defense
SLEP
Shelf-Life Extension Program
SNCO
Staff Non Commission Officer
SON
Statement of Need
T/E
Table of Equipment
TECOM
Training and Education Command
TFSMS
Total Force Structure Management System
TLAMM
Theater Lead Agent for Medical Materiel
TML+
Tactical Medical Logistics Planning Tool
Class VIII Management
11-5
T/O
Table of Organization
TOECR
Table of Organization, Equipment Change Request
UNP
Urgent Needs Process
UNS
Urgent Needs Statement
UUNS
Urgent Universal Needs Statement
USTRANSCOM
United States Transportation Command
USAMMA
US Army Medical Materiel Agency
WIPEB
Warfighting Program Evaluation Board
Class VIII Management
11-6
3. Definitions
Allowance. Authorized quantities of consumable supplies, durables, and
equipment distributed throughout the Marine Expeditionary Force (MEF)
to provide a capability to perform a specific function identified to
support the designated health care mission.
Assemblage. A module with all required AMALs/ADAL to establish a
specific health care capability or to treat a specific population at
risk (PAR). Assemblages have an assigned TAMCN associated with the
capability set.
Authorized Medical Allowance List (AMAL). A list containing the
minimum requirements of materiel which establishes a specific health
care function under combat/deployed operations.
AMAL Supply. A list of consumable supplies that are required to
support a predetermined patient care load associated with a specific
health care function.
Authorized Dental Allowance List (ADAL). A list of the minimum types
and quantities of equipment required to establish a specific dental
care function (e.g., dental operatory/dental clinic) combined with the
list of consumable supplies that are required to support a
predetermined patient care load associated with the dental care
function.
Module. The packaging of equipment or supplies, which comprise a
AMALs/ADAL, into a functional unit.
Defense Medical Management Program Office (DMMPO). The DMMPO is
comprised of a general/flag officer from each of the four military
services. The DMMPO is tasked by the Assistant Secretary of Defense
for Health Affairs (ASD/HA) to develop commonality of deployable
medical facilities and provide recommendations to improve commonality
amongst the services. DMMPO members include Office of the Assistant
Secretary of Defense, Health Affairs (HA); Office of the Joint Chief
of Staff (OJCS/J4), Deputy Director for Medical Readiness; DLA,
Director, J3; Joint Forces Command (JFCOM), Command Surgeon; USARMC;
HQMC, Medical Officer; Office of the Chief of Naval Operations,
Director of Medical Resources, Plans, and Policy Division (N931);
USAF, Assistant Surgeon General.
Standardization. Uniformity on the basis of national stock number
(NSN) or authorized substitutes.
